Millipore6070s
6070s 时间:2021-03-02 阅读:()
JournalofCancer2020,Vol.
11http://www.
jcancer.
org6070JournalofCancer2020;11(20):6070-6080.
doi:10.
7150/jca.
44367ResearchPaperZIC2promotescancerstemcelltraitsviaup-regulatingOCT4expressioninlungadenocarcinomacellsWangWei-Hua1*,ZhouNing1*,ChenQian2andJiangDao-Wen11.
Departmentofthoracicsurgery,MinhangHospital,FudanUniversity,Shanghai201100,China.
2.
Departmentofgeneralsurgery,MinhangHospital,FudanUniversity,Shanghai201100,China.
*Theseauthorscontributedequallytothismanuscript.
Correspondingauthors:JiangDao-Wen,Departmentofthoracicsurgery,MinhangHospital,FudanUniversity,Shanghai201100,China.
Tel.
:8621-64175590,Fax:8621-64175590;E-mail:jiang_daowen1@163.
com;ortoChenQian,Departmentofthoracicsurgery,MinhangHospital,FudanUniversity,Shanghai201100,China,Tel:8621-64175590,Fax:8621-64175590;E-mail:chenqian2010@sohu.
com.
Theauthor(s).
ThisisanopenaccessarticledistributedunderthetermsoftheCreativeCommonsAttributionLicense(https://creativecommons.
org/licenses/by/4.
0/).
Seehttp://ivyspring.
com/termsforfulltermsandconditions.
Received:2020.
01.
29;Accepted:2020.
06.
29;Published:2020.
08.
19AbstractBackground:Accumulatingevidencehasrevealedtheimportanceofcancerstemcells(CSCs)inself-renewalandchemoresistance.
PreviousstudiesreportedhighexpressionofZIC2wascloselyassociatedwithtumorigenesisandCSCtraits.
However,theroleofZIC2asacrucialfactorforregulatingCSCpropertiesinlungadenocarcinoma(LAC)remainselusive.
Methods:RT-PCRandWBassaywereemployedtoassessZIC2expressionin20LACtumortissuesandthematchednon-canceroustissues.
TheroleofZIC2inLACCSCwereanalyzedbyevaluationofCSC-relatedmarkersexpressionandspheroidformationinvitro.
CisplatinandpaclitaxelresistancecapacitieswereevaluatedbyCCK8assay,colonyformationassay,andflowcytometryanalysis.
SubcutaneousNOD/SCIDmicemodelsweregeneratedtoassessinvivoCSCfeatures.
Results:HighexpressionofZIC2wasfoundinLACtumortissuesandindicatedapooroverallsurvivalinLACpatients.
ZIC2upregulatedanarrayofCSCs-relatedgenes,includingEpCAM,OCT4,SOX2,NANOG,C-MycandBmi-1.
KnockdownofZIC2inhibitedsphere-formingcapacityanddecreasedcisplatinandpaclitaxelresistance.
However,overexpressionofZIC2achievedoppositeeffects.
Mechanically,ZIC2actsupstreamofOCT4topromoteitsexpression,resultinginenhancementofCSCtraitsinLAC.
Conclusion:OurresultsdemonstratedthatZIC2wascrucialforpromotingCSCtraitsinLACcells,andservedasapotentialbiomarkerforpredictingprognosis.
TheZIC2-OCT4networkwillfacilitatetheevaluationofthepotentialtherapeuticefficacyofchemotherapyandpredictpatientsensitivitytotreatment.
Keywords:lungadenocarcinoma;cancerstemcells;ZIC2;OCT4activationIntroductionLungcanceristhemajorcauseofcancer-relateddeathsworldwide[1-3].
Lungadenocarcinoma(LAC)islistedasthemostcommonsubtypesoflungcancer,accountingforalmost40%[4-6].
DistantmetastasisandchemoresistanceareprimarilyattributedtoshortsurvivalforLACpatients[7].
Thecancerstemcells(CSCs)aredefinedasararesubsetofcancercellsandpossessesabilitytodifferentiateandself-renew[8].
Theyarealsoresponsiblefortumorgrowth,cancerousrecurrence,metastasisandchemoresistance[9].
Therefore,understandingoftherolesandmechanismscriticaltolungCSCgenerationandexpansionwouldimprovetheoverallsurvivalratesinLACpatients.
Transcriptionfactor-mediatedexpressionregulationisoneofthecriticalregulatorymechanismscontributingtoCSCstraits[10].
OCT4,NANOGandSOX2havebeenidentifiedasthecorepluripotentTFsinembryonicstemcells,whichplayimportantrolesinestablishingandmaintainingthepluripotencyofstemIvyspringInternationalPublisherJournalofCancer2020,Vol.
11http://www.
jcancer.
org6071cells[11-13].
OCT4,amemberofthePOUfamily,exertsafundamentalroleincancerstemcellself-renewalandmultipledifferentiationpotential[14].
PreviousstudiesrevealedthatOCT4waspreferentiallyexpressedinLACstemcellsandshowedcloselycorrelationwiththegenerationandexpansionofLACCSCs[15-17].
Additionally,OCT4iscriticaltoinducedrugresistanceinLACpatients[17].
However,whyOCT4exhibitedhighexpressioninLACstemcellsremainedunclear.
Thus,itisessentialtoexploretheregulatorymechanismsofOCT4expression,whichwouldcontributetounderstandthetumorgenesisanddrugresistanceinLACandobtainmorepotentialtherapeuticbenefitsinclinic.
Thezincfingerfamilymember2(ZIC2)functionsasatranscriptionalregulatorandhasbeenreportedtobehighlyexpressedinsolidtumors[18].
Importantly,highexpressionofZIC2iscloselyassociatedwithtumorigenesisandself-renewalofcancercells[19].
AccumulatingevidenceindicatedZIC2maybeinvolvedinlungcancerprogression[20,21].
Interestingly,ZIC2-dependentOCT4expressionwasinvolvedinregulatingCSCtraitsinlivercancer[19].
However,theroleofZIC2inLACwasstillunknown.
Here,wefoundthatZIC2wasupregulatedinLACtissuesandindicatessignificantlypoorerprognosis.
Additionally,ZIC2greatlyenhancedsphere-forming,proliferationandchemoresistancecapacitiesofLACcells.
Importantly,werevealedthatZIC2wasakeyupstreamregulatorygeneofOCT4,whichsustainsthestemnessofLACCSCs.
MaterialsandMethodsHumantissuesamplesPairedperi-tumorandtumortissuespecimenswereobtainedfrom20LACpatientsintheDepartmentofthoracicsurgery,MinhangHospital,FudanUniversity,forsubsequentexperiments.
Apathologicaldiagnosiswasmadeaccordingtothehistologyoftumorspecimensorbiopsyandwasexaminedbyexperiencedpathologists.
Allparticipantsinvolvedinthisstudyprovidedinformedconsentbeforethecommencementofthestudy.
CelllinesNormallungcellsHBECandhumanLACcelllinesA549,NCI-H1975,PC9,NCI-H1650,NCI-H23,NCI-H1299werepurchasedfromCellBankofTypeCultureCollectionofChineseAcademyofSciences(Shanghai,China).
CellswereculturedinDulbecco'sModifiedEagleMedium(DMEM)(Gibco,USA)containing10%fetalbovineserum(FBS)(Gibco,USA)and100units/mlofpenicillinand100g/mlofstreptomycin(Gibco,USA)inahumidified37°Cincubatorwith5%CO2.
AccordingtotheexpressionofZIC2inlivercelllines,wechosetheZIC2-highexpressingLACcelllineNCI-H1299toperformtheloss-of-functionexperiments.
TheZIC2low-expressingLACcelllineNCI-H1975wasusedtoperformthegain-of-functionexperiments.
EstablishmentofstablecelllinesStablecelllineswereestablishedaspreviouslydescribed[18].
TwoshRNAsequencesspecificallyagainstZIC2(shZIC2#1,shZIC2#2)andcontrol-shRNAwereused.
TwodistinctshRNAHuSH29-mershRNAconstructsagainstZIC2inpGFP-V-RSvec-torwerepurchasedfromMerdobioTechnologies(Shanghai,China).
TheproductionoflentiviralparticlesandsubsequentinfectionofNCI-H1299andNCI-H1975cellswereperformed.
Positivecellswereselectedwithpuromycin(Clontech,MountainView,CA,USA)for10days.
RT-PCRmRNAwasextractedaccordingtostandardprotocolsprovidedbyTRIzol(Invitrogen,USA).
ThetotalmRNAwasreversedtocDNAfollowedbythePrimerScriptTMRTreagentKitwithgDNAEraser(PerfectRealTime)(TaKaRa,China).
Quantitativereal-timePCRwasperformedwithEvaGreen2*qPCRMasterMix(Invitrogen,USA)inaCFX96TMReal-TimePCRSystem(BioRad,USA).
RT-qPCRwasperformedintriplicate.
TherelativelevelsofmRNAwerenormalizedtothoseofGAPDH.
TheprimersequencesusedarelistedinSupplementalTable1.
WesternblotWesternblotwasperformedaspreviouslydescribed.
Indetails,culturedcellswerelysedinRIPAlysisbuffer(Beyotime,China)supplementedwithcocktailproteaseinhibitor(Roche,Switzerland).
ProteinswereseparatedbySDS-PAGEandweretransferredontopolyvinylidenedifluoridemembranes(Millipore,USA).
Themembraneswereblockedin5%nonfatmilksolutionfor1hat37°Candwereincubatedwithprimaryantibodiesat4°Covernight,followedbyincubationwithHRP-conjugatedsecondaryantibodies(Beyotime,China)for1h.
ThesignalswerevisualizedusingtheBeyoECLPluskit(Beyotime,China).
TheantibodiesusedcanbefoundinSupplementalTable2.
GAPDHwasusedasaninternalcontrol.
CellviabilityassayCells(5*103)wereseededin96-wellplatesfor12h,followedbytreatmentwithcisplatin(Sigma,USA)atvariousconcentrations(1,5,10,20,50,100,500,1000M)andpaclitaxelatvariousconcentrations(0.
001,0.
01,0.
1,1,10,100m).
Afterincubationfor48JournalofCancer2020,Vol.
11http://www.
jcancer.
org6072h,cellviabilitywasmeasuredbyboththe3-(4,5-dimethylthiazolyl-2]-2,5-diphenyltetrazoliumbromide(MTT)(Sigma,StLouis,MO,USA)assayandCellCountKit-8(CCK-8)(KeyGENBioTECH,China)assayaccordingtothemanufacturer'sinstructions.
Thepercentageofviablecells(%)=(absorbanceoftreatedsample/absorbanceofuntreatedsample)*100%.
ColonyformingassayCells(400cells/well)wereseededin6-wellplates.
After2weeksofculture,colonieswerefixedby4%paraformaldehydeandwerestainedwith0.
5%crystalviolet.
Thenumberofcolonies(≥100mindiameter)wascountedwithamicroscope.
SphereformingassayNCI-H1299cellsandNCI-H1975cellswereseededinultra-lowattachment6-wellplates(Corning,USA)andculturedinDMEM/F12supplementedwithB27,20ng/mlEGF,20ng/mlbFGF,and10ng/mlHGF.
CellswereincubatedinaCO2incubator2weekslater;sphereswerecountedundermicroscope(Olympus,Japan).
FlowcytometryCellsweretreatedwithdoxorubicin(0.
5g/ml)for48h.
Next,cellswereharvestedandstainedinbindingbuffer,AnnexinV-APCand7-AADasprovidedbytheAnnexinV-APC/7-AADApoptosisDetectionKit(KeyGENBioTECH,China).
Analysiswasdeterminedusingaflowcytometer.
StatisticalanalysisAllexperimentswereconductedatleastintriplicate.
Allthedatawerepresentedasthemeans±standarderrorofthemean(SEM).
StatisticalanalyseswereperformedusingStudent'st-testwithGraphPadPrism6(GraphPadSoftware,USA).
Inallcases,P50%)(Figure2B).
Therefore,wefirstobservedtheeffectsofZIC2onexpressionsofCSC-associatedmarkersincludingEpCAM,OCT4,SOX2,NANOG,C-MycandBmi-1.
ResultsshowedthatZIC2knockdownsignificantlyreducedmRNAexpressionoftheseCSC-associatedmarkerexpressioninNCI-H1299cells(Figure2C).
Consistently,WBassaysconfirmedthefindingsofRT-PCRassays(Figure2D).
Next,theeffectofZIC2expressiononsphere-formingcapacityofLACcellswasassessed.
WefoundthatdownregulationofZIC2greatlydecreasedthenumbersofspheroidcells(Figure2E).
Last,weexaminedeffectsofZIC2onCSCtraitsviaserialtumorigenesisexperiments.
ResultsshowedthatZIC2knockdownobviouslyinhibitedtumorformationatalldensitieswetested(Figure2F),confirmingthecriticalroleofZIC2inmaintainingCSCstraitsinNCI-H1299cells.
KnockdownofZIC2sensitizedLACcellstoconventionalchemotherapyregimensItiswellacknowledgedthatchemoresistanceactsasahallmarkofstemcell-liketrait[22],thus,wealsoinvestigatedtheinvolvementofZIC2indrugresistanceoflungadenocarcinomacells.
CCK8assayswereperformedtoevaluatetheinhibitoryratesofLACcellsunderdifferentdrugconcentrationtreatment.
WefoundthatZIC2knockdownresultedinamoresignificantreducedinhibitionrateswhentreatedwithcisplatininawiderangeofconcentrations(from5μMto500μm),comparedwiththecontrolcells(Figure3A).
Consistently,ZIC2knockdowncellsalsosensitizedNCI-H1299cellstopaclitaxeltreatment(Figure3B).
Tofurtherverifyabovefindings,wechosethetwosignificantinhibitoryconcentrationofcisplatin(10um,20um)andpaclitaxel(0.
01umand0.
1um)totreatLACcells,andcellproliferationpotentialsunderchemotherapyregimentreatmentwereassessedbycolonyformationassays.
WefoundthatZIC2knockdownsensitizedLACcellstoeithercisplatinorpaclitaxeltreatmentatbothtwoconcentrations,evidencedbydecreasednumberofclonesformed(Figure3CandD).
Finally,toconsolidateourpreviousresults,flowcytometryassayswereperformed.
Resultsshowedthat,comparedwithcontrolcells,ZIC2knockdownresultedinsignificantlyhigherapoptosisratesofLACcellswhentreatedwithcertainconcentrationofcisplatinorpaclitaxel(Figure3EandF).
Therefore,ourdatademonstratethatZICknockdownsignificantlysensitizedLACcellstoconventionalchemotherapyregimenttreatments.
OverexpressionofZIC2promotesstemcell-likephenotypeinLACToconfirmtheroleofZIC2inLACCSCmaintenanceandexpansion,wesuccessfullyconstructedthelentiviral-basedstableZIC2over-expressedNCI-H1975cells,confirmedbysignificantlyincreasedZIC2expressionaccordingtoRT-PCRandWBassays(Figure4A).
Afterwards,expressionsofCSC-associatedmarkers,sphere-forming,anddrugresistancecapacitieswerefurtherinvestigated.
ZIC2overexpressionresultedinincreasedexpressionlevelsofEpCAM,OCT4,SOX2,NANOG,C-MycandBmi-1accordingtoresultsofRT-PCRandWBassays(Figure4BandC).
Ofnote,theZIC2-OENCI-H1975cellsshowedanincreasedsphere-formingnumberswhencomparedwithcontrolcells(Figure4D).
Thus,ourdataindicatedthatforcedexpressionofZIC2inLACcellsdrovethesecellstowardsaCSC-likestate.
Finally,resultsfromserialtumorigenesisexperimentsshowedthatZIC2overexpressiongreatlyenhancedtumorformationcapacityofNCI-H1975cellsatalldensitieswetested(Figure2F).
JournalofCancer2020,Vol.
11http://www.
jcancer.
org6074Figure2.
ZIC2knockdownrestrictedCSC-associatedphenotypeinLACcells.
(A)ExpressionlevelsofZIC2innormalandLACcelllinesweredeterminedbyRT-PCRandWBassays.
(B)ZIC2knockdownefficiencieswereevaluatedbyRT-PCR(left)andWB(right)assays.
(C)EffectsofZIC2knockdownonCSC-associatedmarkerexpressioninNCI-H1299cellswereevaluatedbyRT-PCRassays.
(D)WBresultsfordescribingtheeffectsofZIC2knockdownonCSC-associatedmarkerexpressioninNCI-H1299cells.
(E)EffectsofZIC2knockdownonsphere-formingcapacityinNCI-H1299cells;Representativeimageswereshowninleftpanel.
(F)Ratiooftumor-freemiceafter12weeks'tumorformationafterinjectionofindicatednumbersofLACcells.
Imageswereshownintheleftpanel.
JournalofCancer2020,Vol.
11http://www.
jcancer.
org6075Figure3.
ZIC2knockdownsensitizedNCI-H1299cellstocisplatinandpaclitaxeltreatment.
(A)EffectsofZIC2knockdownontheresponsetodifferentconcentrationsofcisplatininNCI-H1299cellswereevaluatedbyCCK-8assays.
(B)EffectsofZIC2knockdownontheresponsetodifferentconcentrationsofpaclitaxelinNCI-H1299cellswereevaluatedbyCCK-8assays.
(C)ColonyformationassayswereconductedtoevaluatetheeffectsofZICknockdownoncisplatinresistanceinNCI-H1299cells;twoconcentrations(10and20mM)wereselectedaccordingtotheresultsofCCK-8assays.
(D)ColonyformationassayswereconductedtoevaluatetheeffectsofZICknockdownonpaclitaxelresistanceinNCI-H1299cells;twoconcentrations(0.
01and1mM)wereselectedaccordingtotheresultsofCCK-8assays.
(E)ApoptosisratesofZIC2-KDandcorrespondingparentalNCI-H1299cellsunderdifferentconcentrationsofcisplatintreatmentsweredeterminedbyFACS.
(F)ApoptosisratesofZIC2-KDandcorrespondingparentalNCI-H1299cellsunderdifferentconcentrationsofpaclitaxeltreatmentsweredeterminedbyFACS.
OverexpressionofZIC2rendersLACcellswithresistancetoconventionalchemotherapyregimensNext,wesetouttoinvestigatetheimpactsofforceexpressionofZIC2ondrugresistance.
CCK8assaysindicatedthatZIC2-overexpressedcellsexhibitedamoresignificanthigherresistancetovariousconcentrationsofcisplatintreatment(from5μMto500μm),comparedwithcontrolcells(Figure5A).
Similarly,ZIC2overexpressionalsosensitizedNCI-H1299cellstodifferentconcentrationsofpaclitaxeltreatment(Figure5B).
Colony-formationassaysindicatedthatZIC2overexpressionconferredconsiderablecisplatinandpaclitaxelresistancecapacitiestoLACcells,evidencedbyincreasednumberofclonesformedundersameregimentreatment(Figure5CandD).
Finally,FACSassaysindicatedthatcomparedwithcontrolcells,ZIC2overexpressionresultedinsignificantlylowerapoptosisratesofLACcellswhentreatedwithcertainconcentrationofcisplatinorpaclitaxel(Figure5EandF).
Takentogether,thesedatademonstratethatforcedZIC2expressionsignificantlysensitizedLACcellstoconventionalchemotherapyregimenttreatments.
ZIC2enhancesCSCtraitsviaanOCT4-dependentmannerAmongthenumerousstemness-relatedmoleculesassociatedwithZIC2expression,wefocusedonOCT4,whichshowedthemostsignificantmRNAexpressionchangesduetoZIC2alterations.
Also,OCT4wasreportedtoplaypivotalroleforCSCpropertiesinLAC[17].
ToinvestigateinteractionofZIC2withOCT4inLACCSCs,weoverexpressedOCT4inZIC2-KDNCI-H1299cells,whilesilencedOCT4expressioninZIC2-OENCI-H1975cells.
Asexpected,forcedexpressionofOCT4restoredexpressionofCSC-associatedmarkersinZIC2-KDNCI-H1299cells,whereasknockingdownOCT4greatlyabolishedthepromotionaleffectsofZIC2overexpressiononCSC-associatedmarkerexpressionJournalofCancer2020,Vol.
11http://www.
jcancer.
org6076(Figure6AandB).
Moreover,OCT4overexpressionsuccessfullyrescuedthesphere-formingcapacityinNCI-H1299cells,evidencedbyincreasednumberofspheroidsformed.
Onthecontrary,OCT4silencedramaticallyattenuatedthesphere-formingcapacityrenderedbyZIC2overexpressioninNCI-H1975cells(Figure6C).
Moreimportantly,upregulationofOCT4reversedtheZIC2knockdown-inducedcisplatinandpaclitaxelsensitizationinNCI-H1299cellsaccordingtoCCK-8assays(Figure6D),whereasdownregulationofOCT4significantlyabolishedZIC2-mediatedcisplatinandpaclitaxelresistanceinNCI-H1975cells(Figure6E).
InconcordancewiththeresultsofCCK-8assay,FACSresultsdemonstratedthattheapoptosisratesofZIC2-KDNCI-H1299cellsundercisplatinorpaclitaxeltreatmentsweresignificantlyreduceduponOCT4overexpression(Figure6F).
Contrarily,OCT4knockdownincreasedtheapoptosisratesinZIC2-OENCI-H1975cellsatbothtwoconcentrations(Figure6G).
Together,ourfindingsstronglyindicatedthatZIC2promotedCSCtraitsinLACviaup-regulatingOCT4expression.
Figure4.
ZIC2overexpressionpromotedCSC-associatedphenotypeinNCI-H1975cells.
(A)EfficienciesofZIC2overexpressioninNCI-H1975cellswerevalidatedbyRT-PCRandWBassays.
(B)EffectsofZIC2overexpressiononCSC-associatedmarkerexpressionweredeterminedbyRT-PCRassays.
(C)EffectsofZIC2overexpressiononCSC-associatedmarkerexpressionweredeterminedbyWBassays.
(D)EffectsofZIC2overexpressiononsphere-formingcapacityinNCI-H1975cellswereevaluated;Representativeimageswereshownasleftpanel.
(F)Ratiooftumor-freemiceafter12weeks'tumorformationafterinjectionofindicatednumbersofLACcells.
Imageswereshownintheleftpanel.
JournalofCancer2020,Vol.
11http://www.
jcancer.
org6077Figure5.
ZIC2overexpressioninducedresistancetocisplatinandpaclitaxelinNCI-H1975cells.
(A)EffectsofZIC2overexpressionontheresponsetodifferentconcentrationsofcisplatininNCI-H1975cellswereevaluatedbyCCK-8assays.
(B)EffectsofZIC2overexpressionontheresponsetodifferentconcentrationsofpaclitaxelinNCI-H1975cellswereevaluatedbyCCK-8assays.
(C)ColonyformationassayswereconductedtoevaluatetheeffectsofZICoverexpressiononcisplatinresistanceinNCI-H1975cells;twoconcentrations(10and20mM)wereselectedaccordingtotheresultsofCCK-8assays.
(D)ColonyformationassayswereconductedtoevaluatetheeffectsofZICoverexpressiononpaclitaxelresistanceinNCI-H1975cells;twoconcentrations(10and20mM)wereselectedaccordingtotheresultsofCCK-8assays.
(E)ApoptosisratesofZIC2-OEandcorrespondingparentalNCI-H1299cellsunderdifferentconcentrationsofcisplatintreatmentsweredeterminedbyFACS.
(F)ApoptosisratesofZIC2-OEandcorrespondingparentalNCI-H1299cellsunderdifferentconcentrationsofpaclitaxeltreatmentsweredeterminedbyFACS.
DiscussionZIC2isageneoriginallyidentifiedbytheirhomologytothedrosophilaodd-pairedgenes.
HeterozygousdeletionsandmutationsofZIC2cancauseseverebrainmalformation[23,24].
Thus,itiscriticalforthedevelopmentofCNS[25].
RecentreportshaveshownacorrelationbetweenZIC2expressionandcarcinogenesis.
ZIC2isaberrantlyactivatedinvariouscancertypes,suchashepatocellularcarcinoma[19],bladdercancer[26],andcervicalcancer[27].
ZIC2isupregulatedinthesetumorsandisassociatedwithahigherstageoftumorsandpoorprognosis.
Therefore,ZIC2isregardedasanoncogene.
However,therearenodataregardingtheexpressionofZIC2inLACanditsclinicalsignificance.
Inourstudy,werevealedthatZIC2washighlyexpressedinLACtumortissuesandindicatedapooroverallsurvivalofLACpatients,whichsuggestedthatZIC2maybeapotentialprognosticbiomarkerforLACpatients.
CSCshavebeenidentifiedinmanysolidtumorsandplayacentralroleintumorinitiation,progression,andtherapeuticresistance[28].
AbetterunderstandingofthemolecularmechanismsessentialforCSCmaintenanceandexpansionwouldshedlightontheenhancementofclinicalmanagement.
Importantly,specificallyandeffectivelytargetingCSCsiswidelyconsideredasapotentialstrategytoimprovetheoverallsurvivalratesofcancerpatients[19,29].
PreviousstudiesrevealedthatZIC2wasrequiredfortheself-renewalmaintenanceofliverCSCs[19].
Additionally,arecentreportshowedthatZIC2ishighlyexpressedincervicalcancerandassociatedwithactivationofHedgehogsignaling,acrucialcancerstemnesspathway[27].
However,theLACCSCbiologyremainslargelyunknownandwhetherZIC2playaroleinLACCSChasnotbeenreportedpreviously.
Here,usingbothloss-of-functionandgain-of-functionexperiments,weprovideevidencefortheroleofZIC2intheregulationofLACcellself-renewalandstemness.
WefoundZIC2expressionwaspositivelyassociatedwiththeexpressionofstemmed-relatedmoleculesandwasessentialforthespheroidcellformation.
JournalofCancer2020,Vol.
11http://www.
jcancer.
org6078Figure6.
ZIC2promotedCSCtraitsinLACvieup-regulatingOCT4expression.
(A)OCT4expressionwasrestoredinZIC2-KDNCI-H1299cells(upper),orsilencedinZIC2-OENCI-H1975cells(lower),andtheirimpactsonCSC-associatedmarkerexpressionwereevaluatedbyRT-CPR.
(B)OCT4expressionwasrestoredinZIC2-KDNCI-H1299cells(left),orsilencedinZIC2-OENCI-H1975cells(right),andtheirimpactsonCSC-associatedmarkerexpressionwereevaluatedbyWBassays.
(C)OCT4expressionwasrestoredinZIC2-KDNCI-H1299cells(upper),orsilencedinZIC2-OENCI-H1975cells(lower),andtheirimpactsonsphere-formingcapacitieswereevaluated.
(D)OCT4expressionwasrestoredinZIC2-KDNCI-H1299cells,anditsimpactsontheresponsetodifferentconcentrationsofcisplatin(left)orpaclitaxel(right)weredetectedbyCCK-8assays.
(E)OCT4expressionwassilencedinZIC2-OENCI-H1975cells,anditsimpactsontheresponsetodifferentconcentrationsofcisplatin(left)orpaclitaxel(right)weredetectedbyCCK-8assays.
(F)OCT4expressionwasrestoredinZIC2-KDNCI-H1299cells,anditsimpactonapoptosisrateunderdifferentconcentrationsofcisplatin(left)orpaclitaxel(right)treatmentwasdeterminedbyFACS.
(G)OCT4expressionwassilencedinZIC2-OENCI-H1975cells,anditsimpactonapoptosisrateunderdifferentconcentrationsofcisplatin(left)orpaclitaxel(right)treatmentwasdeterminedbyFACS.
Thedevelopmentofchemoresistanceisamajorobstaclefortheeffectivetreatmentofhumanmalignancies[30].
Cisplatinandpaclitaxelarefist-linechemotherapeuticagentsusedforvariouscancersJournalofCancer2020,Vol.
11http://www.
jcancer.
org6079[31].
However,theresponsetothesedrugsforLACpatientssoondisappears,leadingtopatientdeathandthepoorfive-yearsurvivalrates[32].
Chemoresistanceisacomplexphenomenonandrevealingthemolecularmechanismsrelatedtoinherentandacquiredresistanceisimportanttoovercomedrugresistance.
ConsideringthatcancerstemcellsareresponsibleforthetumorchemoresistanceandthefunctionalrolesofZIC2inCSCs,theinfluenceofZIC2oncisplatinandpaclitaxelresistanceneededtobedeeplyinvestigatedinlungadenocarcinomacells.
OurstudyfoundthatZIC2overexpressioncellsdramaticallyenhanceddrugresistancepropertiesinLACcells.
Therefore,ourstudyidentifiedanoveltherapeutictargetforreversingdrugresistanceinLAC,anddevelopingstrategytolowerZIC2expressionmayincreasetheefficacyofcisplatinandpaclitaxeltreatmentinLACtumor.
OCT4wasidentifiedasastemcelltranscriptionfactorandhadbeenreportedtobehighlyexpressedinLACtumor[12,17].
OCT4actsasagatekeeperinthebeginningofmammaliandevelopmentandregulatesthetranscriptionofmanygenes,suchasSOX2,NANOGandc-MYC[12].
Indeed,OCT4wasthekeygenebetweentumorinitiationandprogressionviathedirectmediationofkeymoleculesthatregulatecancerstemnesstraits[33].
Moreover,highOCT4expressionwasfoundtobecloselyassociatedwithpooroutcomesinLACpatients[17].
ZIC2,astranscriptionfactor(TF),behavedastheothercorepluripotentTFs(SOX2,NANOG,c-MYC)incancerstemcells[24].
Interestingly,theassociationbetweenZIC2andOCT4isreportedinlivercancer[19].
Inaddition,ZIC2isrequiredfortheexpressionofOCT4.
Notsurprisingly,lungcancersubtypesmayalsosharecoreregulatorygenesandcommonsignalingpathways.
OurinvitroassaysfurtherdemonstratedthatZIC2actedasacriticaloncogeneresponsibleforself-renewalandstemnessofLACbyupregulationofOCT4.
Therefore,ZIC2-inducedOCT4maybeconsideredasamasterregulatortomaintainandgovernLACCSCs.
ThissuggeststhattheregulatorypathwaybetweenZIC2andOCT4maybesignificantforthestabilizationofstemness-likestate.
Insummary,ourstudyprovidedvariousevidencetolinkhighexpressionofZIC2withtheacquisitionofLACCSC-likeproperties,andproposedanovelmechanismofLACCSC-liketraitsmediatedbyZIC2.
WeunveiledthatZIC2maintainedtheself-renewal,stemnessandchemoresistancecapacitiesinanOCT4-dependentmanner.
Toourknowledge,thisisthefirststudytorevealthatZIC2exerteddramaticeffectsonCSC-likepropertiesinhumanLACtumor.
TheZIC2-OCT4networkwillfacilitatetheevaluationofthepotentialtherapeuticefficacyofchemotherapyandpredictpatientsensitivitytotreatment.
SupplementaryMaterialSupplementaryfiguresandtables.
http://www.
jcancer.
org/v11p6070s1.
pdfAcknowledgmentsDataavailabilityThedatasetsusedand/oranalyzedduringthecurrentstudyareavailablefromthecorrespondingauthoronreasonablerequest.
EthicsapprovalandconsenttoparticipateThepresentstudywasapprovedbytheMinhangHospitalResearchEthicsCommittee,andallindividualsprovidedtheirinformedconsent.
Humanecareofanimalswasobjectedtothe"GuidefortheCareandUseofLaboratoryAnimals"criteriaofNationalAcademyofScience(NationalInstituteofHealthpublication86-23,revised1985).
Authors'contributionStudydesign:DWJ,andQC;Acquisition,analysis,andinterpretationofthedata:WHW,NZ,QC,andDWJ;Draftingofthemanuscript:WHW,NZ,QC,andDWJ;Criticalrevisionofthemanuscript:WHW,NZ,QC,andDWJ;Approveofthefinalmanuscript:allauthors.
CompetingInterestsTheauthorshavedeclaredthatnocompetinginterestexists.
References1.
ZhangC,LeighlNB,WuYL,ZhongWZ.
Emergingtherapiesfornon-smallcelllungcancer.
JHEMATOLONCOL2019;12:45.
2.
ZhangYC,ZhouQ,WuYL.
TheemergingrolesofNGS-basedliquidbiopsyinnon-smallcelllungcancer.
JHEMATOLONCOL2017;10:167.
3.
SomasundaramA,BurnsTF.
Thenextgenerationofimmunotherapy:keepinglungcancerincheck.
JHEMATOLONCOL2017;10:87.
4.
DongS,QuX,LiW,ZhongX,LiP,YangS,ChenX,ShaoM,ZhangL.
Thelongnon-codingRNA,GAS5,enhancesgefitinib-inducedcelldeathininnateEGFRtyrosinekinaseinhibitor-resistantlungadenocarcinomacellswithwide-typeEGFRviadownregulationoftheIGF-1Rexpression.
JHEMATOLONCOL2015;8:43.
5.
ZhaoY,WangR,ShenX,PanY,ChengC,LiY,ShenL,ZhangY,LiH,ZhengD,YeT,ZhengS,SunY,ChenH.
MinorComponentsofMicropapillaryandSolidSubtypesinLungAdenocarcinomaarePredictorsofLymphNodeMetastasisandPoorPrognosis.
ANNSURGONCOL2016;23:2099-2105.
6.
YangHC,KimHR,JheonS,KimK,ChoS,AhnS,LeeHY,ChungJH,ChungKY,BaeMK,ParkSY,KimDK,ChoiSH,ZoJI,KimMS,LeeJM,KimJ,ShimYM,NaKJ,YunJS,ParkJY.
RecurrenceRisk-ScoringModelforStageIAdenocarcinomaoftheLung.
ANNSURGONCOL2015;22:4089-4097.
7.
WeiC,DongX,LuH,TongF,ChenL,ZhangR,DongJ,HuY,WuG,DongX.
LPCAT1promotesbrainmetastasisoflungadenocarcinomabyup-regulatingPI3K/AKT/MYCpathway.
JExpClinCancerRes2019;38:95.
8.
SchulenburgA,BlattK,Cerny-ReitererS,SadovnikI,HerrmannH,MarianB,GruntTW,ZielinskiCC,ValentP.
Cancerstemcellsinbasicscienceandintranslationaloncology:canwetranslateintoclinicalapplicationJHEMATOLONCOL2015;8:16.
JournalofCancer2020,Vol.
11http://www.
jcancer.
org60809.
CrawfordHC,PascaDMM,BanerjeeS.
SignalingNetworksThatControlCellularPlasticityinPancreaticTumorigenesis,Progression,andMetastasis.
GASTROENTEROLOGY2019;156:2073-2084.
10.
KaufholdS,GarbanH,BonavidaB.
YinYang1isassociatedwithcancerstemcelltranscriptionfactors(SOX2,OCT4,BMI1)andclinicalimplication.
JExpClinCancerRes2016;35:84.
11.
IvSL,XieX,OldM,TeknosTN,PanQ.
Emergingroleofnanogintumorigenesisandcancerstemcells.
INTJCANCER2014;135:2741-2748.
12.
ZeineddineD,HammoudAA,MortadaM,BoeufH.
TheOct4protein:morethanamagicstemnessmarker.
AmJStemCells2014;3:74-82.
13.
HuserL,NovakD,UmanskyV,AltevogtP,UtikalJ.
TargetingSOX2inanticancertherapy.
ExpertOpinTherTargets2018;22:983-991.
14.
LeeJH,YunCW,HanYS,KimS,JeongD,KwonHY,KimH,BaekMJ,LeeSH.
Melatoninand5-fluorouracilco-suppresscoloncancerstemcellsbyregulatingcellularprionprotein-Oct4axis.
JPINEALRES2018;65:e12519.
15.
FengYH,SuYC,LinSF,LinPR,WuCL,TungCL,LiCF,ShiehGS,ShiauAL.
Oct4upregulatesosteopontinviaEgr1andisassociatedwithpooroutcomeinhumanlungcancer.
BMCCANCER2019;19:791.
16.
MaiuthedA,BhummaphanN,LuanpitpongS,MutiranguraA,AporntewanC,MeeprasertA,RungrotmongkolT,RojanasakulY,ChanvorachoteP.
NitricoxidepromotescancercelldedifferentiationbydisruptinganOct4:caveolin-1complex:Anewregulatorymechanismforcancerstemcellformation.
JBIOLCHEM2018;293:13534-13552.
17.
JenJ,TangYA,LuYH,LinCC,LaiWW,WangYC.
Oct4transcriptionallyregulatestheexpressionoflongnon-codingRNAsNEAT1andMALAT1topromotelungcancerprogression.
MOLCANCER2017;16:104.
18.
LuSX,ZhangCZ,LuoRZ,WangCH,LiuLL,FuJ,ZhangL,WangH,XieD,YunJP.
Zic2promotestumorgrowthandmetastasisviaPAK4inhepatocellularcarcinoma.
CANCERLETT2017;402:71-80.
19.
ZhuP,WangY,HeL,HuangG,DuY,ZhangG,YanX,XiaP,YeB,WangS,HaoL,WuJ,FanZ.
ZIC2-dependentOCT4activationdrivesself-renewalofhumanlivercancerstemcells.
JCLININVEST2015;125:3795-3808.
20.
HanW,ZhangC,GaoXJ,WangHB,ChenF,CaoF,HuYW,MaJ,GuX,DingHZ.
ClinicopathologicandPrognosticSignificanceoftheZincFingeroftheCerebellumFamilyinInvasiveBreastCancer.
JBREASTCANCER2018;21:51-61.
21.
WangYF,YangHY,ShiXQ,WangY.
UpregulationofmicroRNA-129-5pinhibitscellinvasion,migrationandtumorangiogenesisbyinhibitingZIC2viadownregulationoftheHedgehogsignalingpathwayincervicalcancer.
CANCERBIOLTHER2018;19:1162-1173.
22.
MaXL,SunYF,WangBL,ShenMN,ZhouY,ChenJW,HuB,GongZJ,ZhangX,CaoY,PanBS,ZhouJ,FanJ,GuoW,YangXR.
Sphere-formingcultureenricheslivercancerstemcellsandrevealsStearoyl-CoAdesaturase1asapotentialtherapeutictarget.
BMCCANCER2019;19:760.
23.
HuangS,JinA.
ZIC2promotesviabilityandinvasionofhumanosteosarcomacellsbysuppressingSHIP2expressionandactivatingPI3K/AKTpathways.
JCELLBIOCHEM2018;119:2248-2257.
24.
LuoZ,GaoX,LinC,SmithER,MarshallSA,SwansonSK,FlorensL,WashburnMP,ShilatifardA.
Zic2isanenhancer-bindingfactorrequiredforembryonicstemcellspecification.
MOLCELL2015;57:685-694.
25.
EscalanteA,MurilloB,Morenilla-PalaoC,KlarA,HerreraE.
Zic2-dependentaxonmidlineavoidancecontrolstheformationofmajoripsilateraltractsintheCNS.
NEURON2013;80:1392-1406.
26.
WangJ,MaW,LiuY.
Longnon-codingRNAHULCpromotesbladdercancercellsproliferationbutinhibitsapoptosisviaregulationofZIC2andPI3K/AKTsignalingpathway.
CANCERBIOMARK2017;20:425-434.
27.
ChanDW,LiuVW,LeungLY,YaoKM,ChanKK,CheungAN,NganHY.
Zic2synergisticallyenhancesHedgehogsignallingthroughnuclearretentionofGli1incervicalcancercells.
JPATHOL2011;225:525-534.
28.
MaXL,JiangM,ZhaoY,WangBL,ShenMN,ZhouY,ZhangCY,SunYF,ChenJW,HuB,GongZJ,ZhangX,CaoY,PanBS,ZhouJ,FanJ,YangXR,GuoW.
ApplicationofSerumAnnexinA3inDiagnosis,OutcomePredictionandTherapeuticResponseEvaluationforPatientswithHepatocellularCarcinoma.
ANNSURGONCOL2018;25:1686-1694.
29.
MaXL,ShenMN,HuB,WangBL,YangWJ,LvLH,WangH,ZhouY,JinAL,SunYF,ZhangCY,QiuSJ,PanBS,ZhouJ,FanJ,YangXR,GuoW.
CD73promoteshepatocellularcarcinomaprogressionandmetastasisviaactivatingPI3K/AKTsignalingbyinducingRap1-mediatedmembranelocalizationofP110betaandpredictspoorprognosis.
JHEMATOLONCOL2019;12:37.
30.
HuB,ChengJW,HuJW,LiH,MaXL,TangWG,SunYF,GuoW,HuangA,ZhouKQ,GaoPT,CaoY,QiuSJ,ZhouJ,FanJ,YangXR.
KPNA3ConfersSorafenibResistancetoAdvancedHepatocellularCarcinomaviaTWISTRegulatedEpithelial-MesenchymalTransition.
JCANCER2019;10:3914-3925.
31.
ArbourKC,RielyGJ.
SystemicTherapyforLocallyAdvancedandMetastaticNon-SmallCellLungCancer:AReview.
JAMA2019;322:764-774.
32.
KrisMG,GasparLE,ChaftJE,KennedyEB,AzzoliCG,EllisPM,LinSH,PassHI,SethR,ShepherdFA,SpigelDR,StrawnJR,UngYC,WeyantM.
AdjuvantSystemicTherapyandAdjuvantRadiationTherapyforStageItoIIIACompletelyResectedNon-Small-CellLungCancers:AmericanSocietyofClinicalOncology/CancerCareOntarioClinicalPracticeGuidelineUpdate.
JCLINONCOL2017;35:2960-2974.
33.
VillodreES,KipperFC,PereiraMB,LenzG.
RolesofOCT4intumorigenesis,cancertherapyresistanceandprognosis.
CANCERTREATREV2016;51:1-9.
11http://www.
jcancer.
org6070JournalofCancer2020;11(20):6070-6080.
doi:10.
7150/jca.
44367ResearchPaperZIC2promotescancerstemcelltraitsviaup-regulatingOCT4expressioninlungadenocarcinomacellsWangWei-Hua1*,ZhouNing1*,ChenQian2andJiangDao-Wen11.
Departmentofthoracicsurgery,MinhangHospital,FudanUniversity,Shanghai201100,China.
2.
Departmentofgeneralsurgery,MinhangHospital,FudanUniversity,Shanghai201100,China.
*Theseauthorscontributedequallytothismanuscript.
Correspondingauthors:JiangDao-Wen,Departmentofthoracicsurgery,MinhangHospital,FudanUniversity,Shanghai201100,China.
Tel.
:8621-64175590,Fax:8621-64175590;E-mail:jiang_daowen1@163.
com;ortoChenQian,Departmentofthoracicsurgery,MinhangHospital,FudanUniversity,Shanghai201100,China,Tel:8621-64175590,Fax:8621-64175590;E-mail:chenqian2010@sohu.
com.
Theauthor(s).
ThisisanopenaccessarticledistributedunderthetermsoftheCreativeCommonsAttributionLicense(https://creativecommons.
org/licenses/by/4.
0/).
Seehttp://ivyspring.
com/termsforfulltermsandconditions.
Received:2020.
01.
29;Accepted:2020.
06.
29;Published:2020.
08.
19AbstractBackground:Accumulatingevidencehasrevealedtheimportanceofcancerstemcells(CSCs)inself-renewalandchemoresistance.
PreviousstudiesreportedhighexpressionofZIC2wascloselyassociatedwithtumorigenesisandCSCtraits.
However,theroleofZIC2asacrucialfactorforregulatingCSCpropertiesinlungadenocarcinoma(LAC)remainselusive.
Methods:RT-PCRandWBassaywereemployedtoassessZIC2expressionin20LACtumortissuesandthematchednon-canceroustissues.
TheroleofZIC2inLACCSCwereanalyzedbyevaluationofCSC-relatedmarkersexpressionandspheroidformationinvitro.
CisplatinandpaclitaxelresistancecapacitieswereevaluatedbyCCK8assay,colonyformationassay,andflowcytometryanalysis.
SubcutaneousNOD/SCIDmicemodelsweregeneratedtoassessinvivoCSCfeatures.
Results:HighexpressionofZIC2wasfoundinLACtumortissuesandindicatedapooroverallsurvivalinLACpatients.
ZIC2upregulatedanarrayofCSCs-relatedgenes,includingEpCAM,OCT4,SOX2,NANOG,C-MycandBmi-1.
KnockdownofZIC2inhibitedsphere-formingcapacityanddecreasedcisplatinandpaclitaxelresistance.
However,overexpressionofZIC2achievedoppositeeffects.
Mechanically,ZIC2actsupstreamofOCT4topromoteitsexpression,resultinginenhancementofCSCtraitsinLAC.
Conclusion:OurresultsdemonstratedthatZIC2wascrucialforpromotingCSCtraitsinLACcells,andservedasapotentialbiomarkerforpredictingprognosis.
TheZIC2-OCT4networkwillfacilitatetheevaluationofthepotentialtherapeuticefficacyofchemotherapyandpredictpatientsensitivitytotreatment.
Keywords:lungadenocarcinoma;cancerstemcells;ZIC2;OCT4activationIntroductionLungcanceristhemajorcauseofcancer-relateddeathsworldwide[1-3].
Lungadenocarcinoma(LAC)islistedasthemostcommonsubtypesoflungcancer,accountingforalmost40%[4-6].
DistantmetastasisandchemoresistanceareprimarilyattributedtoshortsurvivalforLACpatients[7].
Thecancerstemcells(CSCs)aredefinedasararesubsetofcancercellsandpossessesabilitytodifferentiateandself-renew[8].
Theyarealsoresponsiblefortumorgrowth,cancerousrecurrence,metastasisandchemoresistance[9].
Therefore,understandingoftherolesandmechanismscriticaltolungCSCgenerationandexpansionwouldimprovetheoverallsurvivalratesinLACpatients.
Transcriptionfactor-mediatedexpressionregulationisoneofthecriticalregulatorymechanismscontributingtoCSCstraits[10].
OCT4,NANOGandSOX2havebeenidentifiedasthecorepluripotentTFsinembryonicstemcells,whichplayimportantrolesinestablishingandmaintainingthepluripotencyofstemIvyspringInternationalPublisherJournalofCancer2020,Vol.
11http://www.
jcancer.
org6071cells[11-13].
OCT4,amemberofthePOUfamily,exertsafundamentalroleincancerstemcellself-renewalandmultipledifferentiationpotential[14].
PreviousstudiesrevealedthatOCT4waspreferentiallyexpressedinLACstemcellsandshowedcloselycorrelationwiththegenerationandexpansionofLACCSCs[15-17].
Additionally,OCT4iscriticaltoinducedrugresistanceinLACpatients[17].
However,whyOCT4exhibitedhighexpressioninLACstemcellsremainedunclear.
Thus,itisessentialtoexploretheregulatorymechanismsofOCT4expression,whichwouldcontributetounderstandthetumorgenesisanddrugresistanceinLACandobtainmorepotentialtherapeuticbenefitsinclinic.
Thezincfingerfamilymember2(ZIC2)functionsasatranscriptionalregulatorandhasbeenreportedtobehighlyexpressedinsolidtumors[18].
Importantly,highexpressionofZIC2iscloselyassociatedwithtumorigenesisandself-renewalofcancercells[19].
AccumulatingevidenceindicatedZIC2maybeinvolvedinlungcancerprogression[20,21].
Interestingly,ZIC2-dependentOCT4expressionwasinvolvedinregulatingCSCtraitsinlivercancer[19].
However,theroleofZIC2inLACwasstillunknown.
Here,wefoundthatZIC2wasupregulatedinLACtissuesandindicatessignificantlypoorerprognosis.
Additionally,ZIC2greatlyenhancedsphere-forming,proliferationandchemoresistancecapacitiesofLACcells.
Importantly,werevealedthatZIC2wasakeyupstreamregulatorygeneofOCT4,whichsustainsthestemnessofLACCSCs.
MaterialsandMethodsHumantissuesamplesPairedperi-tumorandtumortissuespecimenswereobtainedfrom20LACpatientsintheDepartmentofthoracicsurgery,MinhangHospital,FudanUniversity,forsubsequentexperiments.
Apathologicaldiagnosiswasmadeaccordingtothehistologyoftumorspecimensorbiopsyandwasexaminedbyexperiencedpathologists.
Allparticipantsinvolvedinthisstudyprovidedinformedconsentbeforethecommencementofthestudy.
CelllinesNormallungcellsHBECandhumanLACcelllinesA549,NCI-H1975,PC9,NCI-H1650,NCI-H23,NCI-H1299werepurchasedfromCellBankofTypeCultureCollectionofChineseAcademyofSciences(Shanghai,China).
CellswereculturedinDulbecco'sModifiedEagleMedium(DMEM)(Gibco,USA)containing10%fetalbovineserum(FBS)(Gibco,USA)and100units/mlofpenicillinand100g/mlofstreptomycin(Gibco,USA)inahumidified37°Cincubatorwith5%CO2.
AccordingtotheexpressionofZIC2inlivercelllines,wechosetheZIC2-highexpressingLACcelllineNCI-H1299toperformtheloss-of-functionexperiments.
TheZIC2low-expressingLACcelllineNCI-H1975wasusedtoperformthegain-of-functionexperiments.
EstablishmentofstablecelllinesStablecelllineswereestablishedaspreviouslydescribed[18].
TwoshRNAsequencesspecificallyagainstZIC2(shZIC2#1,shZIC2#2)andcontrol-shRNAwereused.
TwodistinctshRNAHuSH29-mershRNAconstructsagainstZIC2inpGFP-V-RSvec-torwerepurchasedfromMerdobioTechnologies(Shanghai,China).
TheproductionoflentiviralparticlesandsubsequentinfectionofNCI-H1299andNCI-H1975cellswereperformed.
Positivecellswereselectedwithpuromycin(Clontech,MountainView,CA,USA)for10days.
RT-PCRmRNAwasextractedaccordingtostandardprotocolsprovidedbyTRIzol(Invitrogen,USA).
ThetotalmRNAwasreversedtocDNAfollowedbythePrimerScriptTMRTreagentKitwithgDNAEraser(PerfectRealTime)(TaKaRa,China).
Quantitativereal-timePCRwasperformedwithEvaGreen2*qPCRMasterMix(Invitrogen,USA)inaCFX96TMReal-TimePCRSystem(BioRad,USA).
RT-qPCRwasperformedintriplicate.
TherelativelevelsofmRNAwerenormalizedtothoseofGAPDH.
TheprimersequencesusedarelistedinSupplementalTable1.
WesternblotWesternblotwasperformedaspreviouslydescribed.
Indetails,culturedcellswerelysedinRIPAlysisbuffer(Beyotime,China)supplementedwithcocktailproteaseinhibitor(Roche,Switzerland).
ProteinswereseparatedbySDS-PAGEandweretransferredontopolyvinylidenedifluoridemembranes(Millipore,USA).
Themembraneswereblockedin5%nonfatmilksolutionfor1hat37°Candwereincubatedwithprimaryantibodiesat4°Covernight,followedbyincubationwithHRP-conjugatedsecondaryantibodies(Beyotime,China)for1h.
ThesignalswerevisualizedusingtheBeyoECLPluskit(Beyotime,China).
TheantibodiesusedcanbefoundinSupplementalTable2.
GAPDHwasusedasaninternalcontrol.
CellviabilityassayCells(5*103)wereseededin96-wellplatesfor12h,followedbytreatmentwithcisplatin(Sigma,USA)atvariousconcentrations(1,5,10,20,50,100,500,1000M)andpaclitaxelatvariousconcentrations(0.
001,0.
01,0.
1,1,10,100m).
Afterincubationfor48JournalofCancer2020,Vol.
11http://www.
jcancer.
org6072h,cellviabilitywasmeasuredbyboththe3-(4,5-dimethylthiazolyl-2]-2,5-diphenyltetrazoliumbromide(MTT)(Sigma,StLouis,MO,USA)assayandCellCountKit-8(CCK-8)(KeyGENBioTECH,China)assayaccordingtothemanufacturer'sinstructions.
Thepercentageofviablecells(%)=(absorbanceoftreatedsample/absorbanceofuntreatedsample)*100%.
ColonyformingassayCells(400cells/well)wereseededin6-wellplates.
After2weeksofculture,colonieswerefixedby4%paraformaldehydeandwerestainedwith0.
5%crystalviolet.
Thenumberofcolonies(≥100mindiameter)wascountedwithamicroscope.
SphereformingassayNCI-H1299cellsandNCI-H1975cellswereseededinultra-lowattachment6-wellplates(Corning,USA)andculturedinDMEM/F12supplementedwithB27,20ng/mlEGF,20ng/mlbFGF,and10ng/mlHGF.
CellswereincubatedinaCO2incubator2weekslater;sphereswerecountedundermicroscope(Olympus,Japan).
FlowcytometryCellsweretreatedwithdoxorubicin(0.
5g/ml)for48h.
Next,cellswereharvestedandstainedinbindingbuffer,AnnexinV-APCand7-AADasprovidedbytheAnnexinV-APC/7-AADApoptosisDetectionKit(KeyGENBioTECH,China).
Analysiswasdeterminedusingaflowcytometer.
StatisticalanalysisAllexperimentswereconductedatleastintriplicate.
Allthedatawerepresentedasthemeans±standarderrorofthemean(SEM).
StatisticalanalyseswereperformedusingStudent'st-testwithGraphPadPrism6(GraphPadSoftware,USA).
Inallcases,P50%)(Figure2B).
Therefore,wefirstobservedtheeffectsofZIC2onexpressionsofCSC-associatedmarkersincludingEpCAM,OCT4,SOX2,NANOG,C-MycandBmi-1.
ResultsshowedthatZIC2knockdownsignificantlyreducedmRNAexpressionoftheseCSC-associatedmarkerexpressioninNCI-H1299cells(Figure2C).
Consistently,WBassaysconfirmedthefindingsofRT-PCRassays(Figure2D).
Next,theeffectofZIC2expressiononsphere-formingcapacityofLACcellswasassessed.
WefoundthatdownregulationofZIC2greatlydecreasedthenumbersofspheroidcells(Figure2E).
Last,weexaminedeffectsofZIC2onCSCtraitsviaserialtumorigenesisexperiments.
ResultsshowedthatZIC2knockdownobviouslyinhibitedtumorformationatalldensitieswetested(Figure2F),confirmingthecriticalroleofZIC2inmaintainingCSCstraitsinNCI-H1299cells.
KnockdownofZIC2sensitizedLACcellstoconventionalchemotherapyregimensItiswellacknowledgedthatchemoresistanceactsasahallmarkofstemcell-liketrait[22],thus,wealsoinvestigatedtheinvolvementofZIC2indrugresistanceoflungadenocarcinomacells.
CCK8assayswereperformedtoevaluatetheinhibitoryratesofLACcellsunderdifferentdrugconcentrationtreatment.
WefoundthatZIC2knockdownresultedinamoresignificantreducedinhibitionrateswhentreatedwithcisplatininawiderangeofconcentrations(from5μMto500μm),comparedwiththecontrolcells(Figure3A).
Consistently,ZIC2knockdowncellsalsosensitizedNCI-H1299cellstopaclitaxeltreatment(Figure3B).
Tofurtherverifyabovefindings,wechosethetwosignificantinhibitoryconcentrationofcisplatin(10um,20um)andpaclitaxel(0.
01umand0.
1um)totreatLACcells,andcellproliferationpotentialsunderchemotherapyregimentreatmentwereassessedbycolonyformationassays.
WefoundthatZIC2knockdownsensitizedLACcellstoeithercisplatinorpaclitaxeltreatmentatbothtwoconcentrations,evidencedbydecreasednumberofclonesformed(Figure3CandD).
Finally,toconsolidateourpreviousresults,flowcytometryassayswereperformed.
Resultsshowedthat,comparedwithcontrolcells,ZIC2knockdownresultedinsignificantlyhigherapoptosisratesofLACcellswhentreatedwithcertainconcentrationofcisplatinorpaclitaxel(Figure3EandF).
Therefore,ourdatademonstratethatZICknockdownsignificantlysensitizedLACcellstoconventionalchemotherapyregimenttreatments.
OverexpressionofZIC2promotesstemcell-likephenotypeinLACToconfirmtheroleofZIC2inLACCSCmaintenanceandexpansion,wesuccessfullyconstructedthelentiviral-basedstableZIC2over-expressedNCI-H1975cells,confirmedbysignificantlyincreasedZIC2expressionaccordingtoRT-PCRandWBassays(Figure4A).
Afterwards,expressionsofCSC-associatedmarkers,sphere-forming,anddrugresistancecapacitieswerefurtherinvestigated.
ZIC2overexpressionresultedinincreasedexpressionlevelsofEpCAM,OCT4,SOX2,NANOG,C-MycandBmi-1accordingtoresultsofRT-PCRandWBassays(Figure4BandC).
Ofnote,theZIC2-OENCI-H1975cellsshowedanincreasedsphere-formingnumberswhencomparedwithcontrolcells(Figure4D).
Thus,ourdataindicatedthatforcedexpressionofZIC2inLACcellsdrovethesecellstowardsaCSC-likestate.
Finally,resultsfromserialtumorigenesisexperimentsshowedthatZIC2overexpressiongreatlyenhancedtumorformationcapacityofNCI-H1975cellsatalldensitieswetested(Figure2F).
JournalofCancer2020,Vol.
11http://www.
jcancer.
org6074Figure2.
ZIC2knockdownrestrictedCSC-associatedphenotypeinLACcells.
(A)ExpressionlevelsofZIC2innormalandLACcelllinesweredeterminedbyRT-PCRandWBassays.
(B)ZIC2knockdownefficiencieswereevaluatedbyRT-PCR(left)andWB(right)assays.
(C)EffectsofZIC2knockdownonCSC-associatedmarkerexpressioninNCI-H1299cellswereevaluatedbyRT-PCRassays.
(D)WBresultsfordescribingtheeffectsofZIC2knockdownonCSC-associatedmarkerexpressioninNCI-H1299cells.
(E)EffectsofZIC2knockdownonsphere-formingcapacityinNCI-H1299cells;Representativeimageswereshowninleftpanel.
(F)Ratiooftumor-freemiceafter12weeks'tumorformationafterinjectionofindicatednumbersofLACcells.
Imageswereshownintheleftpanel.
JournalofCancer2020,Vol.
11http://www.
jcancer.
org6075Figure3.
ZIC2knockdownsensitizedNCI-H1299cellstocisplatinandpaclitaxeltreatment.
(A)EffectsofZIC2knockdownontheresponsetodifferentconcentrationsofcisplatininNCI-H1299cellswereevaluatedbyCCK-8assays.
(B)EffectsofZIC2knockdownontheresponsetodifferentconcentrationsofpaclitaxelinNCI-H1299cellswereevaluatedbyCCK-8assays.
(C)ColonyformationassayswereconductedtoevaluatetheeffectsofZICknockdownoncisplatinresistanceinNCI-H1299cells;twoconcentrations(10and20mM)wereselectedaccordingtotheresultsofCCK-8assays.
(D)ColonyformationassayswereconductedtoevaluatetheeffectsofZICknockdownonpaclitaxelresistanceinNCI-H1299cells;twoconcentrations(0.
01and1mM)wereselectedaccordingtotheresultsofCCK-8assays.
(E)ApoptosisratesofZIC2-KDandcorrespondingparentalNCI-H1299cellsunderdifferentconcentrationsofcisplatintreatmentsweredeterminedbyFACS.
(F)ApoptosisratesofZIC2-KDandcorrespondingparentalNCI-H1299cellsunderdifferentconcentrationsofpaclitaxeltreatmentsweredeterminedbyFACS.
OverexpressionofZIC2rendersLACcellswithresistancetoconventionalchemotherapyregimensNext,wesetouttoinvestigatetheimpactsofforceexpressionofZIC2ondrugresistance.
CCK8assaysindicatedthatZIC2-overexpressedcellsexhibitedamoresignificanthigherresistancetovariousconcentrationsofcisplatintreatment(from5μMto500μm),comparedwithcontrolcells(Figure5A).
Similarly,ZIC2overexpressionalsosensitizedNCI-H1299cellstodifferentconcentrationsofpaclitaxeltreatment(Figure5B).
Colony-formationassaysindicatedthatZIC2overexpressionconferredconsiderablecisplatinandpaclitaxelresistancecapacitiestoLACcells,evidencedbyincreasednumberofclonesformedundersameregimentreatment(Figure5CandD).
Finally,FACSassaysindicatedthatcomparedwithcontrolcells,ZIC2overexpressionresultedinsignificantlylowerapoptosisratesofLACcellswhentreatedwithcertainconcentrationofcisplatinorpaclitaxel(Figure5EandF).
Takentogether,thesedatademonstratethatforcedZIC2expressionsignificantlysensitizedLACcellstoconventionalchemotherapyregimenttreatments.
ZIC2enhancesCSCtraitsviaanOCT4-dependentmannerAmongthenumerousstemness-relatedmoleculesassociatedwithZIC2expression,wefocusedonOCT4,whichshowedthemostsignificantmRNAexpressionchangesduetoZIC2alterations.
Also,OCT4wasreportedtoplaypivotalroleforCSCpropertiesinLAC[17].
ToinvestigateinteractionofZIC2withOCT4inLACCSCs,weoverexpressedOCT4inZIC2-KDNCI-H1299cells,whilesilencedOCT4expressioninZIC2-OENCI-H1975cells.
Asexpected,forcedexpressionofOCT4restoredexpressionofCSC-associatedmarkersinZIC2-KDNCI-H1299cells,whereasknockingdownOCT4greatlyabolishedthepromotionaleffectsofZIC2overexpressiononCSC-associatedmarkerexpressionJournalofCancer2020,Vol.
11http://www.
jcancer.
org6076(Figure6AandB).
Moreover,OCT4overexpressionsuccessfullyrescuedthesphere-formingcapacityinNCI-H1299cells,evidencedbyincreasednumberofspheroidsformed.
Onthecontrary,OCT4silencedramaticallyattenuatedthesphere-formingcapacityrenderedbyZIC2overexpressioninNCI-H1975cells(Figure6C).
Moreimportantly,upregulationofOCT4reversedtheZIC2knockdown-inducedcisplatinandpaclitaxelsensitizationinNCI-H1299cellsaccordingtoCCK-8assays(Figure6D),whereasdownregulationofOCT4significantlyabolishedZIC2-mediatedcisplatinandpaclitaxelresistanceinNCI-H1975cells(Figure6E).
InconcordancewiththeresultsofCCK-8assay,FACSresultsdemonstratedthattheapoptosisratesofZIC2-KDNCI-H1299cellsundercisplatinorpaclitaxeltreatmentsweresignificantlyreduceduponOCT4overexpression(Figure6F).
Contrarily,OCT4knockdownincreasedtheapoptosisratesinZIC2-OENCI-H1975cellsatbothtwoconcentrations(Figure6G).
Together,ourfindingsstronglyindicatedthatZIC2promotedCSCtraitsinLACviaup-regulatingOCT4expression.
Figure4.
ZIC2overexpressionpromotedCSC-associatedphenotypeinNCI-H1975cells.
(A)EfficienciesofZIC2overexpressioninNCI-H1975cellswerevalidatedbyRT-PCRandWBassays.
(B)EffectsofZIC2overexpressiononCSC-associatedmarkerexpressionweredeterminedbyRT-PCRassays.
(C)EffectsofZIC2overexpressiononCSC-associatedmarkerexpressionweredeterminedbyWBassays.
(D)EffectsofZIC2overexpressiononsphere-formingcapacityinNCI-H1975cellswereevaluated;Representativeimageswereshownasleftpanel.
(F)Ratiooftumor-freemiceafter12weeks'tumorformationafterinjectionofindicatednumbersofLACcells.
Imageswereshownintheleftpanel.
JournalofCancer2020,Vol.
11http://www.
jcancer.
org6077Figure5.
ZIC2overexpressioninducedresistancetocisplatinandpaclitaxelinNCI-H1975cells.
(A)EffectsofZIC2overexpressionontheresponsetodifferentconcentrationsofcisplatininNCI-H1975cellswereevaluatedbyCCK-8assays.
(B)EffectsofZIC2overexpressionontheresponsetodifferentconcentrationsofpaclitaxelinNCI-H1975cellswereevaluatedbyCCK-8assays.
(C)ColonyformationassayswereconductedtoevaluatetheeffectsofZICoverexpressiononcisplatinresistanceinNCI-H1975cells;twoconcentrations(10and20mM)wereselectedaccordingtotheresultsofCCK-8assays.
(D)ColonyformationassayswereconductedtoevaluatetheeffectsofZICoverexpressiononpaclitaxelresistanceinNCI-H1975cells;twoconcentrations(10and20mM)wereselectedaccordingtotheresultsofCCK-8assays.
(E)ApoptosisratesofZIC2-OEandcorrespondingparentalNCI-H1299cellsunderdifferentconcentrationsofcisplatintreatmentsweredeterminedbyFACS.
(F)ApoptosisratesofZIC2-OEandcorrespondingparentalNCI-H1299cellsunderdifferentconcentrationsofpaclitaxeltreatmentsweredeterminedbyFACS.
DiscussionZIC2isageneoriginallyidentifiedbytheirhomologytothedrosophilaodd-pairedgenes.
HeterozygousdeletionsandmutationsofZIC2cancauseseverebrainmalformation[23,24].
Thus,itiscriticalforthedevelopmentofCNS[25].
RecentreportshaveshownacorrelationbetweenZIC2expressionandcarcinogenesis.
ZIC2isaberrantlyactivatedinvariouscancertypes,suchashepatocellularcarcinoma[19],bladdercancer[26],andcervicalcancer[27].
ZIC2isupregulatedinthesetumorsandisassociatedwithahigherstageoftumorsandpoorprognosis.
Therefore,ZIC2isregardedasanoncogene.
However,therearenodataregardingtheexpressionofZIC2inLACanditsclinicalsignificance.
Inourstudy,werevealedthatZIC2washighlyexpressedinLACtumortissuesandindicatedapooroverallsurvivalofLACpatients,whichsuggestedthatZIC2maybeapotentialprognosticbiomarkerforLACpatients.
CSCshavebeenidentifiedinmanysolidtumorsandplayacentralroleintumorinitiation,progression,andtherapeuticresistance[28].
AbetterunderstandingofthemolecularmechanismsessentialforCSCmaintenanceandexpansionwouldshedlightontheenhancementofclinicalmanagement.
Importantly,specificallyandeffectivelytargetingCSCsiswidelyconsideredasapotentialstrategytoimprovetheoverallsurvivalratesofcancerpatients[19,29].
PreviousstudiesrevealedthatZIC2wasrequiredfortheself-renewalmaintenanceofliverCSCs[19].
Additionally,arecentreportshowedthatZIC2ishighlyexpressedincervicalcancerandassociatedwithactivationofHedgehogsignaling,acrucialcancerstemnesspathway[27].
However,theLACCSCbiologyremainslargelyunknownandwhetherZIC2playaroleinLACCSChasnotbeenreportedpreviously.
Here,usingbothloss-of-functionandgain-of-functionexperiments,weprovideevidencefortheroleofZIC2intheregulationofLACcellself-renewalandstemness.
WefoundZIC2expressionwaspositivelyassociatedwiththeexpressionofstemmed-relatedmoleculesandwasessentialforthespheroidcellformation.
JournalofCancer2020,Vol.
11http://www.
jcancer.
org6078Figure6.
ZIC2promotedCSCtraitsinLACvieup-regulatingOCT4expression.
(A)OCT4expressionwasrestoredinZIC2-KDNCI-H1299cells(upper),orsilencedinZIC2-OENCI-H1975cells(lower),andtheirimpactsonCSC-associatedmarkerexpressionwereevaluatedbyRT-CPR.
(B)OCT4expressionwasrestoredinZIC2-KDNCI-H1299cells(left),orsilencedinZIC2-OENCI-H1975cells(right),andtheirimpactsonCSC-associatedmarkerexpressionwereevaluatedbyWBassays.
(C)OCT4expressionwasrestoredinZIC2-KDNCI-H1299cells(upper),orsilencedinZIC2-OENCI-H1975cells(lower),andtheirimpactsonsphere-formingcapacitieswereevaluated.
(D)OCT4expressionwasrestoredinZIC2-KDNCI-H1299cells,anditsimpactsontheresponsetodifferentconcentrationsofcisplatin(left)orpaclitaxel(right)weredetectedbyCCK-8assays.
(E)OCT4expressionwassilencedinZIC2-OENCI-H1975cells,anditsimpactsontheresponsetodifferentconcentrationsofcisplatin(left)orpaclitaxel(right)weredetectedbyCCK-8assays.
(F)OCT4expressionwasrestoredinZIC2-KDNCI-H1299cells,anditsimpactonapoptosisrateunderdifferentconcentrationsofcisplatin(left)orpaclitaxel(right)treatmentwasdeterminedbyFACS.
(G)OCT4expressionwassilencedinZIC2-OENCI-H1975cells,anditsimpactonapoptosisrateunderdifferentconcentrationsofcisplatin(left)orpaclitaxel(right)treatmentwasdeterminedbyFACS.
Thedevelopmentofchemoresistanceisamajorobstaclefortheeffectivetreatmentofhumanmalignancies[30].
Cisplatinandpaclitaxelarefist-linechemotherapeuticagentsusedforvariouscancersJournalofCancer2020,Vol.
11http://www.
jcancer.
org6079[31].
However,theresponsetothesedrugsforLACpatientssoondisappears,leadingtopatientdeathandthepoorfive-yearsurvivalrates[32].
Chemoresistanceisacomplexphenomenonandrevealingthemolecularmechanismsrelatedtoinherentandacquiredresistanceisimportanttoovercomedrugresistance.
ConsideringthatcancerstemcellsareresponsibleforthetumorchemoresistanceandthefunctionalrolesofZIC2inCSCs,theinfluenceofZIC2oncisplatinandpaclitaxelresistanceneededtobedeeplyinvestigatedinlungadenocarcinomacells.
OurstudyfoundthatZIC2overexpressioncellsdramaticallyenhanceddrugresistancepropertiesinLACcells.
Therefore,ourstudyidentifiedanoveltherapeutictargetforreversingdrugresistanceinLAC,anddevelopingstrategytolowerZIC2expressionmayincreasetheefficacyofcisplatinandpaclitaxeltreatmentinLACtumor.
OCT4wasidentifiedasastemcelltranscriptionfactorandhadbeenreportedtobehighlyexpressedinLACtumor[12,17].
OCT4actsasagatekeeperinthebeginningofmammaliandevelopmentandregulatesthetranscriptionofmanygenes,suchasSOX2,NANOGandc-MYC[12].
Indeed,OCT4wasthekeygenebetweentumorinitiationandprogressionviathedirectmediationofkeymoleculesthatregulatecancerstemnesstraits[33].
Moreover,highOCT4expressionwasfoundtobecloselyassociatedwithpooroutcomesinLACpatients[17].
ZIC2,astranscriptionfactor(TF),behavedastheothercorepluripotentTFs(SOX2,NANOG,c-MYC)incancerstemcells[24].
Interestingly,theassociationbetweenZIC2andOCT4isreportedinlivercancer[19].
Inaddition,ZIC2isrequiredfortheexpressionofOCT4.
Notsurprisingly,lungcancersubtypesmayalsosharecoreregulatorygenesandcommonsignalingpathways.
OurinvitroassaysfurtherdemonstratedthatZIC2actedasacriticaloncogeneresponsibleforself-renewalandstemnessofLACbyupregulationofOCT4.
Therefore,ZIC2-inducedOCT4maybeconsideredasamasterregulatortomaintainandgovernLACCSCs.
ThissuggeststhattheregulatorypathwaybetweenZIC2andOCT4maybesignificantforthestabilizationofstemness-likestate.
Insummary,ourstudyprovidedvariousevidencetolinkhighexpressionofZIC2withtheacquisitionofLACCSC-likeproperties,andproposedanovelmechanismofLACCSC-liketraitsmediatedbyZIC2.
WeunveiledthatZIC2maintainedtheself-renewal,stemnessandchemoresistancecapacitiesinanOCT4-dependentmanner.
Toourknowledge,thisisthefirststudytorevealthatZIC2exerteddramaticeffectsonCSC-likepropertiesinhumanLACtumor.
TheZIC2-OCT4networkwillfacilitatetheevaluationofthepotentialtherapeuticefficacyofchemotherapyandpredictpatientsensitivitytotreatment.
SupplementaryMaterialSupplementaryfiguresandtables.
http://www.
jcancer.
org/v11p6070s1.
pdfAcknowledgmentsDataavailabilityThedatasetsusedand/oranalyzedduringthecurrentstudyareavailablefromthecorrespondingauthoronreasonablerequest.
EthicsapprovalandconsenttoparticipateThepresentstudywasapprovedbytheMinhangHospitalResearchEthicsCommittee,andallindividualsprovidedtheirinformedconsent.
Humanecareofanimalswasobjectedtothe"GuidefortheCareandUseofLaboratoryAnimals"criteriaofNationalAcademyofScience(NationalInstituteofHealthpublication86-23,revised1985).
Authors'contributionStudydesign:DWJ,andQC;Acquisition,analysis,andinterpretationofthedata:WHW,NZ,QC,andDWJ;Draftingofthemanuscript:WHW,NZ,QC,andDWJ;Criticalrevisionofthemanuscript:WHW,NZ,QC,andDWJ;Approveofthefinalmanuscript:allauthors.
CompetingInterestsTheauthorshavedeclaredthatnocompetinginterestexists.
References1.
ZhangC,LeighlNB,WuYL,ZhongWZ.
Emergingtherapiesfornon-smallcelllungcancer.
JHEMATOLONCOL2019;12:45.
2.
ZhangYC,ZhouQ,WuYL.
TheemergingrolesofNGS-basedliquidbiopsyinnon-smallcelllungcancer.
JHEMATOLONCOL2017;10:167.
3.
SomasundaramA,BurnsTF.
Thenextgenerationofimmunotherapy:keepinglungcancerincheck.
JHEMATOLONCOL2017;10:87.
4.
DongS,QuX,LiW,ZhongX,LiP,YangS,ChenX,ShaoM,ZhangL.
Thelongnon-codingRNA,GAS5,enhancesgefitinib-inducedcelldeathininnateEGFRtyrosinekinaseinhibitor-resistantlungadenocarcinomacellswithwide-typeEGFRviadownregulationoftheIGF-1Rexpression.
JHEMATOLONCOL2015;8:43.
5.
ZhaoY,WangR,ShenX,PanY,ChengC,LiY,ShenL,ZhangY,LiH,ZhengD,YeT,ZhengS,SunY,ChenH.
MinorComponentsofMicropapillaryandSolidSubtypesinLungAdenocarcinomaarePredictorsofLymphNodeMetastasisandPoorPrognosis.
ANNSURGONCOL2016;23:2099-2105.
6.
YangHC,KimHR,JheonS,KimK,ChoS,AhnS,LeeHY,ChungJH,ChungKY,BaeMK,ParkSY,KimDK,ChoiSH,ZoJI,KimMS,LeeJM,KimJ,ShimYM,NaKJ,YunJS,ParkJY.
RecurrenceRisk-ScoringModelforStageIAdenocarcinomaoftheLung.
ANNSURGONCOL2015;22:4089-4097.
7.
WeiC,DongX,LuH,TongF,ChenL,ZhangR,DongJ,HuY,WuG,DongX.
LPCAT1promotesbrainmetastasisoflungadenocarcinomabyup-regulatingPI3K/AKT/MYCpathway.
JExpClinCancerRes2019;38:95.
8.
SchulenburgA,BlattK,Cerny-ReitererS,SadovnikI,HerrmannH,MarianB,GruntTW,ZielinskiCC,ValentP.
Cancerstemcellsinbasicscienceandintranslationaloncology:canwetranslateintoclinicalapplicationJHEMATOLONCOL2015;8:16.
JournalofCancer2020,Vol.
11http://www.
jcancer.
org60809.
CrawfordHC,PascaDMM,BanerjeeS.
SignalingNetworksThatControlCellularPlasticityinPancreaticTumorigenesis,Progression,andMetastasis.
GASTROENTEROLOGY2019;156:2073-2084.
10.
KaufholdS,GarbanH,BonavidaB.
YinYang1isassociatedwithcancerstemcelltranscriptionfactors(SOX2,OCT4,BMI1)andclinicalimplication.
JExpClinCancerRes2016;35:84.
11.
IvSL,XieX,OldM,TeknosTN,PanQ.
Emergingroleofnanogintumorigenesisandcancerstemcells.
INTJCANCER2014;135:2741-2748.
12.
ZeineddineD,HammoudAA,MortadaM,BoeufH.
TheOct4protein:morethanamagicstemnessmarker.
AmJStemCells2014;3:74-82.
13.
HuserL,NovakD,UmanskyV,AltevogtP,UtikalJ.
TargetingSOX2inanticancertherapy.
ExpertOpinTherTargets2018;22:983-991.
14.
LeeJH,YunCW,HanYS,KimS,JeongD,KwonHY,KimH,BaekMJ,LeeSH.
Melatoninand5-fluorouracilco-suppresscoloncancerstemcellsbyregulatingcellularprionprotein-Oct4axis.
JPINEALRES2018;65:e12519.
15.
FengYH,SuYC,LinSF,LinPR,WuCL,TungCL,LiCF,ShiehGS,ShiauAL.
Oct4upregulatesosteopontinviaEgr1andisassociatedwithpooroutcomeinhumanlungcancer.
BMCCANCER2019;19:791.
16.
MaiuthedA,BhummaphanN,LuanpitpongS,MutiranguraA,AporntewanC,MeeprasertA,RungrotmongkolT,RojanasakulY,ChanvorachoteP.
NitricoxidepromotescancercelldedifferentiationbydisruptinganOct4:caveolin-1complex:Anewregulatorymechanismforcancerstemcellformation.
JBIOLCHEM2018;293:13534-13552.
17.
JenJ,TangYA,LuYH,LinCC,LaiWW,WangYC.
Oct4transcriptionallyregulatestheexpressionoflongnon-codingRNAsNEAT1andMALAT1topromotelungcancerprogression.
MOLCANCER2017;16:104.
18.
LuSX,ZhangCZ,LuoRZ,WangCH,LiuLL,FuJ,ZhangL,WangH,XieD,YunJP.
Zic2promotestumorgrowthandmetastasisviaPAK4inhepatocellularcarcinoma.
CANCERLETT2017;402:71-80.
19.
ZhuP,WangY,HeL,HuangG,DuY,ZhangG,YanX,XiaP,YeB,WangS,HaoL,WuJ,FanZ.
ZIC2-dependentOCT4activationdrivesself-renewalofhumanlivercancerstemcells.
JCLININVEST2015;125:3795-3808.
20.
HanW,ZhangC,GaoXJ,WangHB,ChenF,CaoF,HuYW,MaJ,GuX,DingHZ.
ClinicopathologicandPrognosticSignificanceoftheZincFingeroftheCerebellumFamilyinInvasiveBreastCancer.
JBREASTCANCER2018;21:51-61.
21.
WangYF,YangHY,ShiXQ,WangY.
UpregulationofmicroRNA-129-5pinhibitscellinvasion,migrationandtumorangiogenesisbyinhibitingZIC2viadownregulationoftheHedgehogsignalingpathwayincervicalcancer.
CANCERBIOLTHER2018;19:1162-1173.
22.
MaXL,SunYF,WangBL,ShenMN,ZhouY,ChenJW,HuB,GongZJ,ZhangX,CaoY,PanBS,ZhouJ,FanJ,GuoW,YangXR.
Sphere-formingcultureenricheslivercancerstemcellsandrevealsStearoyl-CoAdesaturase1asapotentialtherapeutictarget.
BMCCANCER2019;19:760.
23.
HuangS,JinA.
ZIC2promotesviabilityandinvasionofhumanosteosarcomacellsbysuppressingSHIP2expressionandactivatingPI3K/AKTpathways.
JCELLBIOCHEM2018;119:2248-2257.
24.
LuoZ,GaoX,LinC,SmithER,MarshallSA,SwansonSK,FlorensL,WashburnMP,ShilatifardA.
Zic2isanenhancer-bindingfactorrequiredforembryonicstemcellspecification.
MOLCELL2015;57:685-694.
25.
EscalanteA,MurilloB,Morenilla-PalaoC,KlarA,HerreraE.
Zic2-dependentaxonmidlineavoidancecontrolstheformationofmajoripsilateraltractsintheCNS.
NEURON2013;80:1392-1406.
26.
WangJ,MaW,LiuY.
Longnon-codingRNAHULCpromotesbladdercancercellsproliferationbutinhibitsapoptosisviaregulationofZIC2andPI3K/AKTsignalingpathway.
CANCERBIOMARK2017;20:425-434.
27.
ChanDW,LiuVW,LeungLY,YaoKM,ChanKK,CheungAN,NganHY.
Zic2synergisticallyenhancesHedgehogsignallingthroughnuclearretentionofGli1incervicalcancercells.
JPATHOL2011;225:525-534.
28.
MaXL,JiangM,ZhaoY,WangBL,ShenMN,ZhouY,ZhangCY,SunYF,ChenJW,HuB,GongZJ,ZhangX,CaoY,PanBS,ZhouJ,FanJ,YangXR,GuoW.
ApplicationofSerumAnnexinA3inDiagnosis,OutcomePredictionandTherapeuticResponseEvaluationforPatientswithHepatocellularCarcinoma.
ANNSURGONCOL2018;25:1686-1694.
29.
MaXL,ShenMN,HuB,WangBL,YangWJ,LvLH,WangH,ZhouY,JinAL,SunYF,ZhangCY,QiuSJ,PanBS,ZhouJ,FanJ,YangXR,GuoW.
CD73promoteshepatocellularcarcinomaprogressionandmetastasisviaactivatingPI3K/AKTsignalingbyinducingRap1-mediatedmembranelocalizationofP110betaandpredictspoorprognosis.
JHEMATOLONCOL2019;12:37.
30.
HuB,ChengJW,HuJW,LiH,MaXL,TangWG,SunYF,GuoW,HuangA,ZhouKQ,GaoPT,CaoY,QiuSJ,ZhouJ,FanJ,YangXR.
KPNA3ConfersSorafenibResistancetoAdvancedHepatocellularCarcinomaviaTWISTRegulatedEpithelial-MesenchymalTransition.
JCANCER2019;10:3914-3925.
31.
ArbourKC,RielyGJ.
SystemicTherapyforLocallyAdvancedandMetastaticNon-SmallCellLungCancer:AReview.
JAMA2019;322:764-774.
32.
KrisMG,GasparLE,ChaftJE,KennedyEB,AzzoliCG,EllisPM,LinSH,PassHI,SethR,ShepherdFA,SpigelDR,StrawnJR,UngYC,WeyantM.
AdjuvantSystemicTherapyandAdjuvantRadiationTherapyforStageItoIIIACompletelyResectedNon-Small-CellLungCancers:AmericanSocietyofClinicalOncology/CancerCareOntarioClinicalPracticeGuidelineUpdate.
JCLINONCOL2017;35:2960-2974.
33.
VillodreES,KipperFC,PereiraMB,LenzG.
RolesofOCT4intumorigenesis,cancertherapyresistanceandprognosis.
CANCERTREATREV2016;51:1-9.
- Millipore6070s相关文档
- CL01096070s
- 有期徒刑6
美国G口/香港CTG/美国T级超防云/物理机/CDN大促销 1核 1G 24元/月
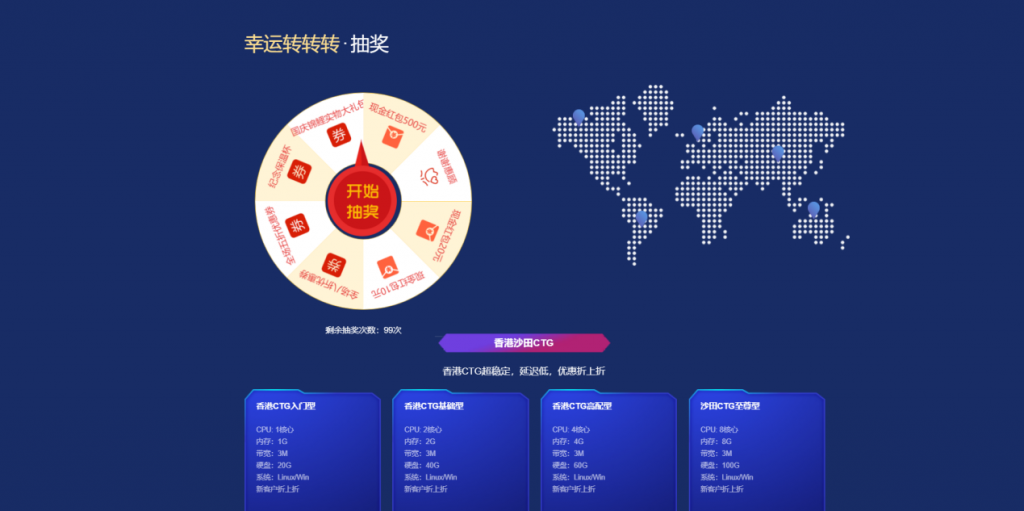
[六一云迎国庆]转盘活动实物礼品美国G口/香港CTG/美国T级超防云/物理机/CDN大促销六一云 成立于2018年,归属于西安六一网络科技有限公司,是一家国内正规持有IDC ISP CDN IRCS电信经营许可证书的老牌商家。大陆持证公司受大陆各部门监管不好用支持退款退现,再也不怕被割韭菜了!主要业务有:国内高防云,美国高防云,美国cera大带宽,香港CTG,香港沙田CN2,海外站群服务,物理机,...
georgedatacenter:美国VPS可选洛杉矶/芝加哥/纽约/达拉斯机房,$20/年;洛杉矶独立服务器39美元/月
georgedatacenter怎么样?georgedatacenter这次其实是两个促销,一是促销一款特价洛杉矶E3-1220 V5独服,性价比其实最高;另外还促销三款特价vps,大家可以根据自己的需要入手。georgedatacenter是一家成立于2019年的美国vps商家,主营美国洛杉矶、芝加哥、达拉斯、新泽西、西雅图机房的VPS、邮件服务器和托管独立服务器业务。georgedatacen...
【IT狗】在线ping,在线tcping,路由追踪
IT狗为用户提供 在线ping、在线tcping、在线路由追踪、域名被墙检测、域名被污染检测 等实用工具。【工具地址】https://www.itdog.cn/【工具特色】1、目前同类网站中,在线ping 仅支持1次或少量次数的测试,无法客观的展现目标服务器一段时间的网络状况,IT狗Ping工具可持续的进行一段时间的ping测试,并生成更为直观的网络质量柱状图,让用户更容易掌握服务器在各地区、各线...
6070s为你推荐
-
免费建站系统免费建站免费wap建站免费手机建站免费自助建站永久免费WAP手机自助建站免费空间免费建站空间怎么改ip怎么改IP地址邮箱怎么写正确的邮箱格式怎么写安装程序配置服务器失败安装用友T3出现安装程序配置服务器失败是怎么回事sourcegear请问高手这是什么“dynamsoft sourceanywhere for vss”,做项目的时候用的,我是新手不知道这是干什么。显卡温度多少正常显卡温度多少算正常?镜像文件是什么系统镜像是什么9flashIE9flash模块异常。硬盘人克隆一个人需要多少人多长时间啊bt封杀BT下载可以封杀迅雷吗?什么原理?能破吗?