reactantswww
www.xvideos.com 时间:2021-03-01 阅读:()
AfacileandconvenientmethodforsynthesisofalkylthiocyanatesunderhomogeneousphasetransfercatalystconditionsAliRezaKiasat*,RashidBadri,SoheilSayyahiChemistryDepartment,CollegeofScience,ShahidChamranUniversity,Ahvaz61357-4-3169,IranReceived15May2008AbstractAsimpleandenvironmentallyfriendlymethodisdescribedfortheefcientconversionofalkylhalidetoalkylthiocyanateusingtetrabutylammoniumbromide(TBAB)asaphasetransfercatalyst.
Thereactionsoccurinwaterandfurnishthecorrespondingalkylthiocyanateinhighyields.
Noevidencefortheformationofisothiocyanatesasby-productofthereactionwasobservedandtheproductswereobtainedinpureformwithoutfurtherpurication.
#2008AliRezaKiasat.
PublishedbyElsevierB.
V.
onbehalfofChineseChemicalSociety.
Allrightsreserved.
Keywords:Alkylthiocyanate;Alkylhalide;Tetrabutylammoniumbromide;Phasetransfercatalyst;SynthesisofalkylthiocyanatesItiswellknownthatthealkylthiocyanate[1]playsanimportantroleasanintermediateforthepreparationofsulfur-containingorganiccompounds[2].
Theyhavefoundawidevarietyofapplicationsasinsecticides,biocidal,antiasthmaticandstartingmaterialsforthepreparationofheterocycles.
Thiocyanatesarealsoconsideredtobeanimportantclassofcompoundsfoundinsomeanticancernaturalproductsformedbydeglycosylationofglucosinolatesderivedfromcruciferousvegetables[3].
Moreover,a-thiocyanatocarbonylcompoundsareintermediatesforapreferredsyntheticroutetoseveraltypesofthiazoles[4].
Thiocyanationisgenerallycarriedoutvianucleophilicsubstitutionusingthiocyanateanions.
Anumberofmethodsareavailableforthepreparationofalkylthiocyanates,suchas,displacementofleavinggroupswiththiocyanateions[5–7].
Thiocyanatescanalsobeobtainedfromalcohols[8],silylethers[9]oramines[10].
However,thisdisplacementfrequentlyrequiresratherseverereactionconditionsbecauseofthelownucleophilicityoftheNCSion.
Hencemanydrawbacksandlowyieldshavebeenobservedforthesethiocyanationmethodologies[2,11].
Inaddition,thethiocyanategroupispoorlystablewhenheatedorunderacidicconditions.
Chromatographyonsilicagelorprolongedheatingover508Ccancauseintramolecularrearrangementtothethermodynamicallyfavoredisothiocyanateisomers[12].
Consequently,thedevelopmentofnewmethodsthataremoreefcientandleadtoconvenientproceduresandbetteryieldsisdesirable.
Phasetransfercatalysts(PTCs)arepowerfulreagentsinchemicaltransformations[13,14],thecharacteristicsofwhichincludemildreactionconditions,safety,operationalsimplicityandselectivity.
PTCsareoftenusedinnucleophilicdisplacementreactionstofacilitatereactionsbetweenorganicreactantsandionicinorganicsalts[15].
www.
elsevier.
com/locate/ccletAvailableonlineatwww.
sciencedirect.
comChineseChemicalLetters19(2008)1301–1304*Correspondingauthor.
E-mailaddress:akiasat@scu.
ac.
ir(A.
R.
Kiasat).
1001-8417/$–seefrontmatter#2008AliRezaKiasat.
PublishedbyElsevierB.
V.
onbehalfofChineseChemicalSociety.
Allrightsreserved.
doi:10.
1016/j.
cclet.
2008.
07.
019Althoughmanyphasetransfercatalystsareknown,quaternarysaltsformedfromammoniaarepracticallyimportantandusedinmanyoforganicreactions.
Incontinuationofourinvestigationsonnewmethodologiesforthesynthesisoforgano-sulfurcompounds[16],wereportanovelandhighlyefcientprotocolthatallowstherapidsynthesisofalkylthiocyanatesusingTBABasaphasetransfercatalystundermildandhomogeneouscondition(Scheme1).
Itshighpolarityandabilitytosolubilizebothorganicandinorganiccompoundscanresultinenhancedreactionratesandcanprovidehigherselectivitycomparedtoconventionalmethods[17,18].
1.
ExperimentalAll1Hand13CNMRdatawererecordedonaBrukerAdvancedDPX400MHzinstrumentspectrometerusingMe4SiastheinternalstandardinCDCl3.
IRspectrawererecordedonaBOMEMMB-Series1998FT-IRspectrometer.
ThepuritydeterminationoftheproductsandreactionmonitoringwereaccomplishedbyTLConsilicagelpolygramSILG/UV254plates.
Generalprocedure:Inatypicalprocedure,amixtureofalkylhalide(1.
0mmol),TBAB(0.
5mmol),KSCN(1.
5mmol)andwater(3.
0mL)wasplacedinaaskandstirredatroomtemperatureorheated(408C)forthetimespeciedinTable1.
Oncompletionofthereaction,followedbyTLCexamination,themixtureallowedtobecoldandextractedintoether(35mL).
Thecombinedorganiclayerwashedwithcoldwater(310mL),driedoversodiumsulfateandltered.
Theltratewassubjectedtoavacuumgavethedesiredproduct(75–95%).
Itdidnotrequireanycolumnchromatographythusavoidingthepossibilityofrearrangement.
AllofthereactionsreportedherewerecleanasjudgedbyTLC,FTIRandNMRanalysisofthecrudereactionmixture.
2.
ResultsanddiscussionsInarstsetofexperiments,awell-stirredsolutionof4-bromobenzylbromide(1.
0mmol)inH2O(3.
0mL)wastreatedwithKSCN(1-2mmol)atroomtemperatureinthepresenceoftetrabutylammoniumbromide(0.
2–0.
5mmol)asaphasetransfercatalyst.
ThereactionwasmonitoredbyTLC.
Aftersomeexperiments,itwasfoundthattheuseof1.
5equivofKSCNperalkylhalideinthepresenceofTBAB(0.
5equiv)inwaterwerethebestconditionsandafterstirringfor45minatroomtemperature,thecleanformationofaproductwithlowerRfvaluewasobserved.
WeexaminedthecatalyticabilityofTBABforconversionofalkylhalidestoalkylthiocyanatewithKSCNinwateratroomtemperature.
Thiscatalystactedveryefcientlyanditconvertsdifferentalkylhalidestotheircorrespondingalkylthiocyanatesinhighisolatedyields.
TheobtainedresultsofthereactionaregiveninTable1.
ItisnoteworthythatnoevidencefortheformationofIsothiocyanatesasby-productofthereactionwasobservedandtheproductswereobtainedinpureformwithoutfurtherpurication.
13CresonanceoftheSCNandNCSgroupsat$111and$145ppm,respectively,areverycharacteristicforthiocyanateandisothiocyanatefunctionalities[19].
AsshowninTable1(entries7and8),thissimplemethodcanbeefcientlyusedforpreparationofa-thiocyanatocarbonylcompounds.
AsexpectedtheeffectsofreactantstructureontherateofBimolecularnucleo-philicsubstitution(SN2)reactionshaveappearedinentry12asnoproductwasobservedeven8hofvariouslystirring.
Inconclusion,wehavedevelopedanoptimizedfacilethiocyanationunderphasetransfercatalyst,whichrequiresonlyamoderatereactiontemperatureinwater.
Theadvantagesofpresentprotocol,suchascleanreactionproles,shortreactiontimes,simplicityinoperationandthelowcostofreagentsmakethisnewprocessanattractivealternativetocurrentmethodologies.
A.
R.
Kiasatetal.
/ChineseChemicalLetters19(2008)1301–13041302Scheme1.
A.
R.
Kiasatetal.
/ChineseChemicalLetters19(2008)1301–13041303Table1ConversionofalkylhalidetoalkylthiocyanateunderPTCinH2OEntryAlkylhalideProductTime(min)Yielda,b(%)1608526080330904458554580c64590c7458086080c94580c1018075113095c,d12–8e–aTheNMRandFTIRspectraofallsynthesizedalkylthiocyanatesareinaccordwiththeliterature[2,4,11].
bIsolatedyields.
cThereactionwasperformedat408C.
dGCanalysis.
e8h.
AcknowledgmentPartialsupportforthisworkbyChamranUniversityResearchCouncilisgratefullyacknowledged.
References[1]R.
G.
Guy,in:S.
Patai(Ed.
),TheChemistryoftheCyanatesandTheirThioDerivatives,WileyInterscience,NewYork,1977,p.
819.
[2]O.
Prakash,H.
Kaur,H.
Batra,N.
Rani,S.
P.
Singh,R.
M.
Moriarty,J.
Org.
Chem.
66(2001)2019.
[3]F.
Shahidi,in:C.
J.
Mussinan,M.
E.
Keelan(Eds.
),SulphurCompoundsinFoods,AmericanChemicalSociety,Washington,DC,1994,p.
106.
[4]Y.
Ju,D.
Kumar,R.
S.
Varma,J.
Org.
Chem.
71(2006)6697.
[5]R.
G.
R.
Bacon,in:N.
Kharasch(Ed.
),OrganicSulfurCompounds,PergamonPress,NewYork,1961,p.
304.
[6]J.
W.
Pavlik,P.
Tongcharoensirikul,N.
P.
Bird,A.
C.
Day,J.
A.
Barltrop,J.
Am.
Chem.
Soc.
116(1994)2292.
[7]T.
Sasaki,A.
Nakanishi,M.
Ohno,J.
Org.
Chem.
469(1981)5445.
[8]Y.
Tamura,T.
Kawasaki,M.
Adachi,M.
Tanio,Y.
Kita,TetrahedronLett.
18(1977)4417.
[9]N.
Iranpoor,H.
Firouzabadi,H.
Shaterian,Synlett(2000)65.
[10]P.
Molina,M.
Alajarin,A.
Ferao,M.
J.
Lindon,P.
M.
Fresneda,M.
J.
Vilaplana,Synthesis(1982)472.
[11]P.
Y.
Renard,H.
Schwebel,P.
Vayron,E.
Leclerc,S.
Diasa,C.
Mioskowskia,TetrahedronLett.
42(2001)8479.
[12]R.
G.
R.
Bacon,R.
G.
Guy,J.
Chem.
Soc.
2428(1961)2436.
[13]T.
Takido,M.
Toriyama,K.
Yamashita,T.
Suwa,M.
Seno,Phosphorus,Sulfur,andSilicon178(2003)319.
[14]Z.
H.
Weng,J.
Y.
Wang,X.
G.
Jian,Chin.
Chem.
Lett.
18(2007)936.
[15]M.
J.
ODonnell,AsymmetricPhaseTransferReactions,in:I.
Ojima(Ed.
),CatalyticAsymmetricSynthesis,VerlagChemie,NewYork,2000.
[16]A.
R.
Kiasat,B.
Mokhtari,A.
Savari,F.
Kazemi,Phosphorus,Sulfur,andSilicon183(2008)178.
[17]S.
Mallakpour,Z.
Raee,Eur.
Polym.
J.
43(2007)1510.
[18]B.
X.
Tang,F.
Wang,J.
H.
Li,Y.
X.
Xie,M.
B.
Zhang,J.
Org.
Chem.
72(2007)6294.
[19]N.
Iranpoor,H.
Firouzabadi,N.
Nowrouzi,Tetrahedron62(2006)5498.
A.
R.
Kiasatetal.
/ChineseChemicalLetters19(2008)1301–13041304
Thereactionsoccurinwaterandfurnishthecorrespondingalkylthiocyanateinhighyields.
Noevidencefortheformationofisothiocyanatesasby-productofthereactionwasobservedandtheproductswereobtainedinpureformwithoutfurtherpurication.
#2008AliRezaKiasat.
PublishedbyElsevierB.
V.
onbehalfofChineseChemicalSociety.
Allrightsreserved.
Keywords:Alkylthiocyanate;Alkylhalide;Tetrabutylammoniumbromide;Phasetransfercatalyst;SynthesisofalkylthiocyanatesItiswellknownthatthealkylthiocyanate[1]playsanimportantroleasanintermediateforthepreparationofsulfur-containingorganiccompounds[2].
Theyhavefoundawidevarietyofapplicationsasinsecticides,biocidal,antiasthmaticandstartingmaterialsforthepreparationofheterocycles.
Thiocyanatesarealsoconsideredtobeanimportantclassofcompoundsfoundinsomeanticancernaturalproductsformedbydeglycosylationofglucosinolatesderivedfromcruciferousvegetables[3].
Moreover,a-thiocyanatocarbonylcompoundsareintermediatesforapreferredsyntheticroutetoseveraltypesofthiazoles[4].
Thiocyanationisgenerallycarriedoutvianucleophilicsubstitutionusingthiocyanateanions.
Anumberofmethodsareavailableforthepreparationofalkylthiocyanates,suchas,displacementofleavinggroupswiththiocyanateions[5–7].
Thiocyanatescanalsobeobtainedfromalcohols[8],silylethers[9]oramines[10].
However,thisdisplacementfrequentlyrequiresratherseverereactionconditionsbecauseofthelownucleophilicityoftheNCSion.
Hencemanydrawbacksandlowyieldshavebeenobservedforthesethiocyanationmethodologies[2,11].
Inaddition,thethiocyanategroupispoorlystablewhenheatedorunderacidicconditions.
Chromatographyonsilicagelorprolongedheatingover508Ccancauseintramolecularrearrangementtothethermodynamicallyfavoredisothiocyanateisomers[12].
Consequently,thedevelopmentofnewmethodsthataremoreefcientandleadtoconvenientproceduresandbetteryieldsisdesirable.
Phasetransfercatalysts(PTCs)arepowerfulreagentsinchemicaltransformations[13,14],thecharacteristicsofwhichincludemildreactionconditions,safety,operationalsimplicityandselectivity.
PTCsareoftenusedinnucleophilicdisplacementreactionstofacilitatereactionsbetweenorganicreactantsandionicinorganicsalts[15].
www.
elsevier.
com/locate/ccletAvailableonlineatwww.
sciencedirect.
comChineseChemicalLetters19(2008)1301–1304*Correspondingauthor.
E-mailaddress:akiasat@scu.
ac.
ir(A.
R.
Kiasat).
1001-8417/$–seefrontmatter#2008AliRezaKiasat.
PublishedbyElsevierB.
V.
onbehalfofChineseChemicalSociety.
Allrightsreserved.
doi:10.
1016/j.
cclet.
2008.
07.
019Althoughmanyphasetransfercatalystsareknown,quaternarysaltsformedfromammoniaarepracticallyimportantandusedinmanyoforganicreactions.
Incontinuationofourinvestigationsonnewmethodologiesforthesynthesisoforgano-sulfurcompounds[16],wereportanovelandhighlyefcientprotocolthatallowstherapidsynthesisofalkylthiocyanatesusingTBABasaphasetransfercatalystundermildandhomogeneouscondition(Scheme1).
Itshighpolarityandabilitytosolubilizebothorganicandinorganiccompoundscanresultinenhancedreactionratesandcanprovidehigherselectivitycomparedtoconventionalmethods[17,18].
1.
ExperimentalAll1Hand13CNMRdatawererecordedonaBrukerAdvancedDPX400MHzinstrumentspectrometerusingMe4SiastheinternalstandardinCDCl3.
IRspectrawererecordedonaBOMEMMB-Series1998FT-IRspectrometer.
ThepuritydeterminationoftheproductsandreactionmonitoringwereaccomplishedbyTLConsilicagelpolygramSILG/UV254plates.
Generalprocedure:Inatypicalprocedure,amixtureofalkylhalide(1.
0mmol),TBAB(0.
5mmol),KSCN(1.
5mmol)andwater(3.
0mL)wasplacedinaaskandstirredatroomtemperatureorheated(408C)forthetimespeciedinTable1.
Oncompletionofthereaction,followedbyTLCexamination,themixtureallowedtobecoldandextractedintoether(35mL).
Thecombinedorganiclayerwashedwithcoldwater(310mL),driedoversodiumsulfateandltered.
Theltratewassubjectedtoavacuumgavethedesiredproduct(75–95%).
Itdidnotrequireanycolumnchromatographythusavoidingthepossibilityofrearrangement.
AllofthereactionsreportedherewerecleanasjudgedbyTLC,FTIRandNMRanalysisofthecrudereactionmixture.
2.
ResultsanddiscussionsInarstsetofexperiments,awell-stirredsolutionof4-bromobenzylbromide(1.
0mmol)inH2O(3.
0mL)wastreatedwithKSCN(1-2mmol)atroomtemperatureinthepresenceoftetrabutylammoniumbromide(0.
2–0.
5mmol)asaphasetransfercatalyst.
ThereactionwasmonitoredbyTLC.
Aftersomeexperiments,itwasfoundthattheuseof1.
5equivofKSCNperalkylhalideinthepresenceofTBAB(0.
5equiv)inwaterwerethebestconditionsandafterstirringfor45minatroomtemperature,thecleanformationofaproductwithlowerRfvaluewasobserved.
WeexaminedthecatalyticabilityofTBABforconversionofalkylhalidestoalkylthiocyanatewithKSCNinwateratroomtemperature.
Thiscatalystactedveryefcientlyanditconvertsdifferentalkylhalidestotheircorrespondingalkylthiocyanatesinhighisolatedyields.
TheobtainedresultsofthereactionaregiveninTable1.
ItisnoteworthythatnoevidencefortheformationofIsothiocyanatesasby-productofthereactionwasobservedandtheproductswereobtainedinpureformwithoutfurtherpurication.
13CresonanceoftheSCNandNCSgroupsat$111and$145ppm,respectively,areverycharacteristicforthiocyanateandisothiocyanatefunctionalities[19].
AsshowninTable1(entries7and8),thissimplemethodcanbeefcientlyusedforpreparationofa-thiocyanatocarbonylcompounds.
AsexpectedtheeffectsofreactantstructureontherateofBimolecularnucleo-philicsubstitution(SN2)reactionshaveappearedinentry12asnoproductwasobservedeven8hofvariouslystirring.
Inconclusion,wehavedevelopedanoptimizedfacilethiocyanationunderphasetransfercatalyst,whichrequiresonlyamoderatereactiontemperatureinwater.
Theadvantagesofpresentprotocol,suchascleanreactionproles,shortreactiontimes,simplicityinoperationandthelowcostofreagentsmakethisnewprocessanattractivealternativetocurrentmethodologies.
A.
R.
Kiasatetal.
/ChineseChemicalLetters19(2008)1301–13041302Scheme1.
A.
R.
Kiasatetal.
/ChineseChemicalLetters19(2008)1301–13041303Table1ConversionofalkylhalidetoalkylthiocyanateunderPTCinH2OEntryAlkylhalideProductTime(min)Yielda,b(%)1608526080330904458554580c64590c7458086080c94580c1018075113095c,d12–8e–aTheNMRandFTIRspectraofallsynthesizedalkylthiocyanatesareinaccordwiththeliterature[2,4,11].
bIsolatedyields.
cThereactionwasperformedat408C.
dGCanalysis.
e8h.
AcknowledgmentPartialsupportforthisworkbyChamranUniversityResearchCouncilisgratefullyacknowledged.
References[1]R.
G.
Guy,in:S.
Patai(Ed.
),TheChemistryoftheCyanatesandTheirThioDerivatives,WileyInterscience,NewYork,1977,p.
819.
[2]O.
Prakash,H.
Kaur,H.
Batra,N.
Rani,S.
P.
Singh,R.
M.
Moriarty,J.
Org.
Chem.
66(2001)2019.
[3]F.
Shahidi,in:C.
J.
Mussinan,M.
E.
Keelan(Eds.
),SulphurCompoundsinFoods,AmericanChemicalSociety,Washington,DC,1994,p.
106.
[4]Y.
Ju,D.
Kumar,R.
S.
Varma,J.
Org.
Chem.
71(2006)6697.
[5]R.
G.
R.
Bacon,in:N.
Kharasch(Ed.
),OrganicSulfurCompounds,PergamonPress,NewYork,1961,p.
304.
[6]J.
W.
Pavlik,P.
Tongcharoensirikul,N.
P.
Bird,A.
C.
Day,J.
A.
Barltrop,J.
Am.
Chem.
Soc.
116(1994)2292.
[7]T.
Sasaki,A.
Nakanishi,M.
Ohno,J.
Org.
Chem.
469(1981)5445.
[8]Y.
Tamura,T.
Kawasaki,M.
Adachi,M.
Tanio,Y.
Kita,TetrahedronLett.
18(1977)4417.
[9]N.
Iranpoor,H.
Firouzabadi,H.
Shaterian,Synlett(2000)65.
[10]P.
Molina,M.
Alajarin,A.
Ferao,M.
J.
Lindon,P.
M.
Fresneda,M.
J.
Vilaplana,Synthesis(1982)472.
[11]P.
Y.
Renard,H.
Schwebel,P.
Vayron,E.
Leclerc,S.
Diasa,C.
Mioskowskia,TetrahedronLett.
42(2001)8479.
[12]R.
G.
R.
Bacon,R.
G.
Guy,J.
Chem.
Soc.
2428(1961)2436.
[13]T.
Takido,M.
Toriyama,K.
Yamashita,T.
Suwa,M.
Seno,Phosphorus,Sulfur,andSilicon178(2003)319.
[14]Z.
H.
Weng,J.
Y.
Wang,X.
G.
Jian,Chin.
Chem.
Lett.
18(2007)936.
[15]M.
J.
ODonnell,AsymmetricPhaseTransferReactions,in:I.
Ojima(Ed.
),CatalyticAsymmetricSynthesis,VerlagChemie,NewYork,2000.
[16]A.
R.
Kiasat,B.
Mokhtari,A.
Savari,F.
Kazemi,Phosphorus,Sulfur,andSilicon183(2008)178.
[17]S.
Mallakpour,Z.
Raee,Eur.
Polym.
J.
43(2007)1510.
[18]B.
X.
Tang,F.
Wang,J.
H.
Li,Y.
X.
Xie,M.
B.
Zhang,J.
Org.
Chem.
72(2007)6294.
[19]N.
Iranpoor,H.
Firouzabadi,N.
Nowrouzi,Tetrahedron62(2006)5498.
A.
R.
Kiasatetal.
/ChineseChemicalLetters19(2008)1301–13041304
- reactantswww相关文档
- www.xvideos.com请问www.****.com.hk 和www.****.com.cn一样吗?
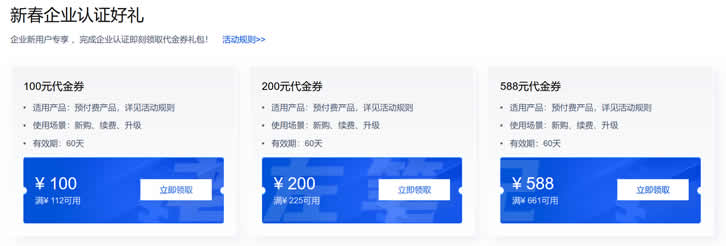
2022年腾讯云新春采购季代金券提前领 领取满减优惠券和域名优惠
2022年春节假期陆续结束,根据惯例在春节之后各大云服务商会继续开始一年的促销活动。今年二月中旬会开启新春采购季的活动,我们已经看到腾讯云商家在春节期间已经有预告活动。当时已经看到有抢先优惠促销活动,目前我们企业和个人可以领取腾讯云代金券满减活动,以及企业用户可以领取域名优惠低至.COM域名1元。 直达链接 - 腾讯云新春采购活动抢先看活动时间:2022年1月20日至2022年2月15日我们可以在...
Megalayer(月599元)限时8月香港和美国大带宽服务器
第一、香港服务器机房这里我们可以看到有提供四个大带宽方案,是全向带宽和国际带宽,前者适合除了中国大陆地区的全网地区用户可以用,后者国际带宽适合欧美地区业务。如果我们是需要大陆地区速度CN2优化的,那就需要选择常规的优化带宽方案,参考这里。CPU内存硬盘带宽流量价格选择E3-12308GB240GB SSD50M全向带宽不限999元/月方案选择E3-12308GB240GB SSD100M国际带宽不...

水墨云历史黑名单IDC,斟酌选购
水墨云怎么样?本站黑名单idc,有被删除账号风险,建议转出及数据备份!水墨云ink cloud Service是成立于2017年的商家,自2020起开始从事香港、日本、韩国、美国等地区CN2 GIA线路的虚拟服务器租赁,同时还有台湾、国内nat vps相关业务,也有iplc专线产品,相对来说主打的是大带宽服务器产品。注意:本站黑名单IDC,有被删除账号风险,请尽量避免,如果已经购买建议转出及数据备...
www.xvideos.com为你推荐
-
摩根币摩根币是什么意思?2020双十一成绩单如何查找2020年小考六年级的成绩?地图应用谁知道什么地图软件好用,求 最好可以看到路上行人原代码什么是原代码haole018.comhttp://www.haoledy.com/view/32092.html 轩辕剑天之痕11、12集在线观看103838.com39052.com这电影网支持网页观看吗?m.kan84.net经常使用http://www.feikan.cc看电影的进来帮我下啊www.kaspersky.com.cn卡巴斯基杀毒软件有免费的吗?稳定版的怎么找?555sss.com不能在线播放了??555woshiheida这个左下角水印woshiheida的gif出处在哪呢?急!!!!!