practicesoray花生壳
oray花生壳 时间:2021-04-03 阅读:()
U.
S.
Food&DrugAdministration10903NewHampshireAvenueDocID#04017.
02.
12SilverSpring,MD20993www.
fda.
govAugust17,2018GENORAYCo.
,Ltd.
℅Ms.
KaitlynnMinBusinessDevelopmentGENORAYAmericaInc.
147E.
BristolLaneORANGECA92780Re:K181943Trade/DeviceName:OSCAR(OSCARPrime,OSCARClassic)RegulationNumber:21CFR892.
1650RegulationName:Image-intensifiedfluoroscopicx-raysystemRegulatoryClass:IIProductCode:OWB,JAADated:July5,2018Received:July20,2018DearMs.
Min:WehavereviewedyourSection510(k)premarketnotificationofintenttomarketthedevicereferencedaboveandhavedeterminedthedeviceissubstantiallyequivalent(fortheindicationsforusestatedintheenclosure)tolegallymarketedpredicatedevicesmarketedininterstatecommercepriortoMay28,1976,theenactmentdateoftheMedicalDeviceAmendments,ortodevicesthathavebeenreclassifiedinaccordancewiththeprovisionsoftheFederalFood,Drug,andCosmeticAct(Act)thatdonotrequireapprovalofapremarketapprovalapplication(PMA).
Youmay,therefore,marketthedevice,subjecttothegeneralcontrolsprovisionsoftheAct.
Althoughthisletterreferstoyourproductasadevice,pleasebeawarethatsomeclearedproductsmayinsteadbecombinationproducts.
The510(k)PremarketNotificationDatabaselocatedathttps://www.
accessdata.
fda.
gov/scripts/cdrh/cfdocs/cfpmn/pmn.
cfmidentifiescombinationproductsubmissions.
ThegeneralcontrolsprovisionsoftheActincluderequirementsforannualregistration,listingofdevices,goodmanufacturingpractice,labeling,andprohibitionsagainstmisbrandingandadulteration.
Pleasenote:CDRHdoesnotevaluateinformationrelatedtocontractliabilitywarranties.
Weremindyou,however,thatdevicelabelingmustbetruthfulandnotmisleading.
Ifyourdeviceisclassified(seeabove)intoeitherclassII(SpecialControls)orclassIII(PMA),itmaybesubjecttoadditionalcontrols.
ExistingmajorregulationsaffectingyourdevicecanbefoundintheCodeofFederalRegulations,Title21,Parts800to898.
Inaddition,FDAmaypublishfurtherannouncementsconcerningyourdeviceintheFederalRegister.
PleasebeadvisedthatFDA'sissuanceofasubstantialequivalencedeterminationdoesnotmeanthatFDAhasmadeadeterminationthatyourdevicecomplieswithotherrequirementsoftheActoranyFederalPage2–Ms.
KaitlynnMinK181943statutesandregulationsadministeredbyotherFederalagencies.
YoumustcomplywithalltheAct'srequirements,including,butnotlimitedto:registrationandlisting(21CFRPart807);labeling(21CFRPart801);medicaldevicereporting(reportingofmedicaldevice-relatedadverseevents)(21CFR803)fordevicesorpostmarketingsafetyreporting(21CFR4,SubpartB)forcombinationproducts(seehttps://www.
fda.
gov/CombinationProducts/GuidanceRegulatoryInformation/ucm597488.
htm);goodmanufacturingpracticerequirementsassetforthinthequalitysystems(QS)regulation(21CFRPart820)fordevicesorcurrentgoodmanufacturingpractices(21CFR4,SubpartA)forcombinationproducts;and,ifapplicable,theelectronicproductradiationcontrolprovisions(Sections531-542oftheAct);21CFR1000-1050.
Also,pleasenotetheregulationentitled,"Misbrandingbyreferencetopremarketnotification"(21CFRPart807.
97).
ForquestionsregardingthereportingofadverseeventsundertheMDRregulation(21CFRPart803),pleasegotohttp://www.
fda.
gov/MedicalDevices/Safety/ReportaProblem/default.
htm.
Forcomprehensiveregulatoryinformationaboutmedicaldevicesandradiation-emittingproducts,includinginformationaboutlabelingregulations,pleaseseeDeviceAdvice(https://www.
fda.
gov/MedicalDevices/DeviceRegulationandGuidance/)andCDRHLearn(http://www.
fda.
gov/Training/CDRHLearn).
Additionally,youmaycontacttheDivisionofIndustryandConsumerEducation(DICE)toaskaquestionaboutaspecificregulatorytopic.
SeetheDICEwebsite(http://www.
fda.
gov/DICE)formoreinformationorcontactDICEbyemail(DICE@fda.
hhs.
gov)orphone(1-800-638-2041or301-796-7100).
Sincerely,RobertOchs,Ph.
D.
DirectorDivisionofRadiologicalHealthOfficeofInVitroDiagnosticsandRadiologicalHealthCenterforDevicesandRadiologicalHealthEnclosurenGjUSGsUX^VXZY`jvumpklu{phsK181943Exhibit5510(k)SummaryDateofSummaryPreparation:July.
05,20181.
SubmitterandUSOfficialCorrespondentSubmitter:GENORAYCo.
,Ltd.
Address:512,560,Dunchon-daero,Jungwon-gu,Seongnam-si,Gyeonggi-Do,KoreaTelephoneNo.
:+82-31-740-4100Fax:+82-31-737-8018OfficialCorrespondent(U.
S):KaitlynnMin-BusinessManagerCorrespondent:GENORAYAmericaInc.
Address:147E.
BristolLane,Orange,CA92865USATelephoneNo.
:+1-855-436-6729Fax:+1-714-786-8919Email:kaitlynn@genorayamerica.
com2.
EstablishmentRegistrationNumber30058434183.
DeviceInformationTrade/DeviceName:OSCAROSCARPrime,OSCARClassicRegulationName:Image-IntensifiedfluoroscopicX-raySystemClassificationName:InterventionalFluoroscopicX-RaySystemProductCode:OWB/InterventionalFluoroscopicX-RaySystemSubsequenceproductcode:JAA/System,X-Ray,Fluoroscopic,Image-IntensifiedDeviceClass:ClassIIperregulation21CFR892.
16504.
PredicateDevice(EquivalentLegallyMarketedDevice)Manufacturer:GENORAYCo.
,LtdDeviceName:ZEN-2090Pro510(k)Number:K091918(DecisionDate–October07,2009)ClassificationName:Image–IntensifiedFluoroscopicX-RaySystemPrimaryProductCode:OWB/InterventionalFluoroscopicX-raySystemSecondaryproductcode:JAA/system,x-ray,fluoroscopic,image-intensifiedOXO/image-intensifiedfluoroscopicx-raysystem,mobileDeviceClass:ClassIIperregulation21CFR892.
16505.
DescriptionoftheDeviceOSCARPrimeandOSCARClassicareclassifiedaccordingtotheoptionofimageacquisitionparts.
FlatpaneldetectorisOSCARPrime,andImageintensifierisOSCARClassic.
AndtheyarecalledOSCARasthebrandname.
OSCARisconsistofX-rayTube,X-raytubeassembly,x-raycontroller,imagereceptorandsomeaccessories.
Thereisnowirelessfunctioninthisdevice.
TheOSCAR,C-ArmMobileisthedeviceintendedtovisualizeanatomicalstructuresbyconvertingapatternofx-radiationintoavisibleimagethroughelectronicamplification.
Thisdeviceisusedforprovidingfluoroscopicandradiographicimagesofpatientanatomy,especiallyduringthespecialproceduresinahospitalormedicalclinics.
Thefluoroscopicmodeofoperationisveryusefultotheattendingphysiciantoseetheimagesonrealtimewithouttheneedtodevelopindividualfilms.
6.
IndicationsforuseOSCARisamobilefluoroscopysystemdesignedtoprovidefluoroscopicandspotfilmimagesofthepatientduringdiagnostic,surgicalandinterventionalprocedures.
Examplesofclinicalapplicationmayincludecholangiography,endoscopy,urologic,orthopedic,neurologic,vascular,andstonelocalization.
ThepatientgroupusingOSCARisallexceptchildren.
7.
SubstantialequivalencechartNameProposeddeviceOSCARPredicatedeviceZEN-2090ProManufacturerGENORAYCo.
,Ltd.
GENORAYCo.
,Ltd.
510(k)No.
-K091918IndicationsforuseOSCARisamobilefluoroscopysystemdesignedtoprovidefluoroscopicandspotfilmimagesofthepatientduringdiagnostic,surgicalandinterventionalprocedures.
Examplesofclinicalapplicationmayincludecholangiography,endoscopy,urologic,orthopedic,neurologic,vascular,cardiacandcriticalcare.
ThepatientgroupusingOSCARisallexceptchildren.
ZEN-2090ProisamobiledigitalC-armdesignedtoprovidefluoroscopicandradiographicimagesofthepatientduringdiagnostic,surgicalandinterventionalprocedures.
Examplesofclinicalapplicationmayincludecholangiography,endoscopy,orthopedic,neurologic,stonelocalization,criticalcareandemergencyroomproceduresi.
e.
surgicalinterventionsneedingX-rayimagingand/orguidanceandinterventionsinsideandoutsidetheoperatingroom.
GeneratorHighFrequencyInverterHighFrequencyInverterMax.
outputpower4.
5kW2.
2kWX-raytubeStationarytubeStationarytubeLarge:1.
4/Small:0.
5Large:1.
5mm/Small:0.
5mmDetectorOSCARPrime(Flatpanel)OSCARClassic(Imageintensifier)ZEN-2090Pro(ImageIntensifier)OptionA:GFD-200(DualRay-Q)(ClearedunderK172180)Activeimagearea:260x256mmCentralResolution:4.
6lp/mmType:CMOSResolution:2600x2560Pixelsamplingresolution:14bitsPixelpitch:100μmMTF:56%DQE:59%Scintillator:CsIOptionA:E5830SD-P4A(ClearedunderK140041)Activeimagearea:9"(9/6/4.
5)Min.
CentralResolutionatthemonitor:-9"(23cm):2.
2lp/mm-6"(15cm):2.
8lp/mm-6"(15cm):3.
0lp/mmDQE:65%(typically)E5830SD-P4AActiveimagearea:9"(9/6/4.
5)Min.
CentralResolutionatthemonitor:-9"(23cm):2.
2lp/mm-6"(15cm):2.
8lp/mm-6"(15cm):3.
0lp/mmDQE:65%(typically)OptionB:VIVIX-D0909GActiveimagearea:229.
12x229.
12mmCentralResolution:2.
7lp/mmType:a-SiTFTResolution:2600x2560Pixelsamplingresolution:16bitPixelpitch:179μmMTF:45%DQE:45%Scintillator:CsIOptionB:TH9438QX(ClearedunderK140041).
Activeimagearea:9"(9/6/4.
5)Min.
CentralResolutionatthemonitor:-9"(23cm):2.
2lp/mm-6"(15cm):2.
8lp/mm-6"(15cm):3.
0lp/mmDQE:65%(typically)Fluoroscopy40-120kV/0.
2-6.
0mA40-110kV/0.
2-6.
0mAPulsedFluoroscopy0.
2-10.
0mA0.
2-6.
0mARadiography40-120kV/0.
6-200mAs40-110kV/0.
4-100mAsDimensionsSID:1000mmSID:960mmPanningRotation:±12.
5PanningRotation:±12.
5OrbitalRotation:155OrbitalRotation:120Vert.
Travel:500mmVert.
Travel:400mmHoriz.
Travel:200mmHoriz.
Travel:200mmTheproposedOSCARisbasedonthepredicatedevice,ZEN-2090Pro(K091918).
ThereareseveraldifferencebetweenOSCAR(Prime,Classic)andpredicatedevice(ZEN-2090Pro)istheoptionsofImageacquisitionparts.
OSCARPrimeistheflatpaneldetectorandpredicatedeviceisImageIntensifier.
Asmentionedinthecomparisontable,predicatedeviceDQEishigherthanOSCARPrime.
However,predicatedeviceDQEistheDQEoftheimageintensifieritself.
Ingeneral,whenimageintensifieriscombinedwithCCDcameratheDQEdecreases.
Inconclusion,theDQEofthecompletepredicatedeviceis51%.
SotheDQEoftheOSCARPrimeismoreeffectiveandsafetythanpredicatedevice.
Alsoflatpaneldetectortypehasexcellentimageuniformity,nogeometricdistortion,noveilingglareorvignetting,smallandthinphysicalsizeascomparedtotheImageIntensifiertype.
TheOSCARissubstantiallyequivalenttothepredicatedevice,ZEN-2090Pro(K091918).
8.
Safety,EMCandPerformancedatacomparisontoPredicateOSCARcomplieswithindustrystandardssuchasIEC60601-1Seriesand21CFR1020.
30,21CFR1020.
31and21CFR1020.
32tominimizeelectrical,mechanicalandradiationhazards.
-Electrical,mechanical,environmentalsafetyandperformancetestingaccordingtostandardIEC60601-1,IEC60601-1-3,IEC60601-1-6,IEC60601-2-28,IEC60601-2-43,IEC60601-2-54andIEC62366wereperformed.
-EMCtestingwasconductedinaccordancewithstandardIEC60601-1-2.
-OSCARmeetstheEPRCstandards(21CFR1020.
30,31,32).
-FDAguidance"guidanceforSSXIdevices",and"guidancefortheContentofPremarketSubmissionsforSoftwareContainedinMedicaldevices",wasperformedforOSCAR.
Thesoftwarefeatureshasbeenchangedcomparetopredicatedeviceasbelow:EnhanceImageCalibrationfunction.
DisplayexposuremodesshowsontheLCDtouchpanel.
AddAngiographyfunctions.
Changestothepredicatedevicesoftwareweretestedandtheydonotaffectthedevicesafetyandeffectiveness.
Also,thedevicesoftwareismoderatelevelofconcern.
Asaresults,alltestresultsweresatisfactoryandtheresultofbenchandclinicalevaluationindicatesthatthenewdeviceisassafeandeffectiveasthepredicatedevice.
9.
ConclusionInreferencetothecomparisoninformationprovidedinsubstantialequivalencechart,andthemostoffunctionsandelectronicfeaturesaresimilarwithpredicatedevice.
WebelievethattheOSCARissafeandeffectiveaspredicatedevice,andhasnonewindicationforuse.
Therefore,OSCARissubstantiallyequivalenttopredicatedevice.
S.
Food&DrugAdministration10903NewHampshireAvenueDocID#04017.
02.
12SilverSpring,MD20993www.
fda.
govAugust17,2018GENORAYCo.
,Ltd.
℅Ms.
KaitlynnMinBusinessDevelopmentGENORAYAmericaInc.
147E.
BristolLaneORANGECA92780Re:K181943Trade/DeviceName:OSCAR(OSCARPrime,OSCARClassic)RegulationNumber:21CFR892.
1650RegulationName:Image-intensifiedfluoroscopicx-raysystemRegulatoryClass:IIProductCode:OWB,JAADated:July5,2018Received:July20,2018DearMs.
Min:WehavereviewedyourSection510(k)premarketnotificationofintenttomarketthedevicereferencedaboveandhavedeterminedthedeviceissubstantiallyequivalent(fortheindicationsforusestatedintheenclosure)tolegallymarketedpredicatedevicesmarketedininterstatecommercepriortoMay28,1976,theenactmentdateoftheMedicalDeviceAmendments,ortodevicesthathavebeenreclassifiedinaccordancewiththeprovisionsoftheFederalFood,Drug,andCosmeticAct(Act)thatdonotrequireapprovalofapremarketapprovalapplication(PMA).
Youmay,therefore,marketthedevice,subjecttothegeneralcontrolsprovisionsoftheAct.
Althoughthisletterreferstoyourproductasadevice,pleasebeawarethatsomeclearedproductsmayinsteadbecombinationproducts.
The510(k)PremarketNotificationDatabaselocatedathttps://www.
accessdata.
fda.
gov/scripts/cdrh/cfdocs/cfpmn/pmn.
cfmidentifiescombinationproductsubmissions.
ThegeneralcontrolsprovisionsoftheActincluderequirementsforannualregistration,listingofdevices,goodmanufacturingpractice,labeling,andprohibitionsagainstmisbrandingandadulteration.
Pleasenote:CDRHdoesnotevaluateinformationrelatedtocontractliabilitywarranties.
Weremindyou,however,thatdevicelabelingmustbetruthfulandnotmisleading.
Ifyourdeviceisclassified(seeabove)intoeitherclassII(SpecialControls)orclassIII(PMA),itmaybesubjecttoadditionalcontrols.
ExistingmajorregulationsaffectingyourdevicecanbefoundintheCodeofFederalRegulations,Title21,Parts800to898.
Inaddition,FDAmaypublishfurtherannouncementsconcerningyourdeviceintheFederalRegister.
PleasebeadvisedthatFDA'sissuanceofasubstantialequivalencedeterminationdoesnotmeanthatFDAhasmadeadeterminationthatyourdevicecomplieswithotherrequirementsoftheActoranyFederalPage2–Ms.
KaitlynnMinK181943statutesandregulationsadministeredbyotherFederalagencies.
YoumustcomplywithalltheAct'srequirements,including,butnotlimitedto:registrationandlisting(21CFRPart807);labeling(21CFRPart801);medicaldevicereporting(reportingofmedicaldevice-relatedadverseevents)(21CFR803)fordevicesorpostmarketingsafetyreporting(21CFR4,SubpartB)forcombinationproducts(seehttps://www.
fda.
gov/CombinationProducts/GuidanceRegulatoryInformation/ucm597488.
htm);goodmanufacturingpracticerequirementsassetforthinthequalitysystems(QS)regulation(21CFRPart820)fordevicesorcurrentgoodmanufacturingpractices(21CFR4,SubpartA)forcombinationproducts;and,ifapplicable,theelectronicproductradiationcontrolprovisions(Sections531-542oftheAct);21CFR1000-1050.
Also,pleasenotetheregulationentitled,"Misbrandingbyreferencetopremarketnotification"(21CFRPart807.
97).
ForquestionsregardingthereportingofadverseeventsundertheMDRregulation(21CFRPart803),pleasegotohttp://www.
fda.
gov/MedicalDevices/Safety/ReportaProblem/default.
htm.
Forcomprehensiveregulatoryinformationaboutmedicaldevicesandradiation-emittingproducts,includinginformationaboutlabelingregulations,pleaseseeDeviceAdvice(https://www.
fda.
gov/MedicalDevices/DeviceRegulationandGuidance/)andCDRHLearn(http://www.
fda.
gov/Training/CDRHLearn).
Additionally,youmaycontacttheDivisionofIndustryandConsumerEducation(DICE)toaskaquestionaboutaspecificregulatorytopic.
SeetheDICEwebsite(http://www.
fda.
gov/DICE)formoreinformationorcontactDICEbyemail(DICE@fda.
hhs.
gov)orphone(1-800-638-2041or301-796-7100).
Sincerely,RobertOchs,Ph.
D.
DirectorDivisionofRadiologicalHealthOfficeofInVitroDiagnosticsandRadiologicalHealthCenterforDevicesandRadiologicalHealthEnclosurenGjUSGsUX^VXZY`jvumpklu{phsK181943Exhibit5510(k)SummaryDateofSummaryPreparation:July.
05,20181.
SubmitterandUSOfficialCorrespondentSubmitter:GENORAYCo.
,Ltd.
Address:512,560,Dunchon-daero,Jungwon-gu,Seongnam-si,Gyeonggi-Do,KoreaTelephoneNo.
:+82-31-740-4100Fax:+82-31-737-8018OfficialCorrespondent(U.
S):KaitlynnMin-BusinessManagerCorrespondent:GENORAYAmericaInc.
Address:147E.
BristolLane,Orange,CA92865USATelephoneNo.
:+1-855-436-6729Fax:+1-714-786-8919Email:kaitlynn@genorayamerica.
com2.
EstablishmentRegistrationNumber30058434183.
DeviceInformationTrade/DeviceName:OSCAROSCARPrime,OSCARClassicRegulationName:Image-IntensifiedfluoroscopicX-raySystemClassificationName:InterventionalFluoroscopicX-RaySystemProductCode:OWB/InterventionalFluoroscopicX-RaySystemSubsequenceproductcode:JAA/System,X-Ray,Fluoroscopic,Image-IntensifiedDeviceClass:ClassIIperregulation21CFR892.
16504.
PredicateDevice(EquivalentLegallyMarketedDevice)Manufacturer:GENORAYCo.
,LtdDeviceName:ZEN-2090Pro510(k)Number:K091918(DecisionDate–October07,2009)ClassificationName:Image–IntensifiedFluoroscopicX-RaySystemPrimaryProductCode:OWB/InterventionalFluoroscopicX-raySystemSecondaryproductcode:JAA/system,x-ray,fluoroscopic,image-intensifiedOXO/image-intensifiedfluoroscopicx-raysystem,mobileDeviceClass:ClassIIperregulation21CFR892.
16505.
DescriptionoftheDeviceOSCARPrimeandOSCARClassicareclassifiedaccordingtotheoptionofimageacquisitionparts.
FlatpaneldetectorisOSCARPrime,andImageintensifierisOSCARClassic.
AndtheyarecalledOSCARasthebrandname.
OSCARisconsistofX-rayTube,X-raytubeassembly,x-raycontroller,imagereceptorandsomeaccessories.
Thereisnowirelessfunctioninthisdevice.
TheOSCAR,C-ArmMobileisthedeviceintendedtovisualizeanatomicalstructuresbyconvertingapatternofx-radiationintoavisibleimagethroughelectronicamplification.
Thisdeviceisusedforprovidingfluoroscopicandradiographicimagesofpatientanatomy,especiallyduringthespecialproceduresinahospitalormedicalclinics.
Thefluoroscopicmodeofoperationisveryusefultotheattendingphysiciantoseetheimagesonrealtimewithouttheneedtodevelopindividualfilms.
6.
IndicationsforuseOSCARisamobilefluoroscopysystemdesignedtoprovidefluoroscopicandspotfilmimagesofthepatientduringdiagnostic,surgicalandinterventionalprocedures.
Examplesofclinicalapplicationmayincludecholangiography,endoscopy,urologic,orthopedic,neurologic,vascular,andstonelocalization.
ThepatientgroupusingOSCARisallexceptchildren.
7.
SubstantialequivalencechartNameProposeddeviceOSCARPredicatedeviceZEN-2090ProManufacturerGENORAYCo.
,Ltd.
GENORAYCo.
,Ltd.
510(k)No.
-K091918IndicationsforuseOSCARisamobilefluoroscopysystemdesignedtoprovidefluoroscopicandspotfilmimagesofthepatientduringdiagnostic,surgicalandinterventionalprocedures.
Examplesofclinicalapplicationmayincludecholangiography,endoscopy,urologic,orthopedic,neurologic,vascular,cardiacandcriticalcare.
ThepatientgroupusingOSCARisallexceptchildren.
ZEN-2090ProisamobiledigitalC-armdesignedtoprovidefluoroscopicandradiographicimagesofthepatientduringdiagnostic,surgicalandinterventionalprocedures.
Examplesofclinicalapplicationmayincludecholangiography,endoscopy,orthopedic,neurologic,stonelocalization,criticalcareandemergencyroomproceduresi.
e.
surgicalinterventionsneedingX-rayimagingand/orguidanceandinterventionsinsideandoutsidetheoperatingroom.
GeneratorHighFrequencyInverterHighFrequencyInverterMax.
outputpower4.
5kW2.
2kWX-raytubeStationarytubeStationarytubeLarge:1.
4/Small:0.
5Large:1.
5mm/Small:0.
5mmDetectorOSCARPrime(Flatpanel)OSCARClassic(Imageintensifier)ZEN-2090Pro(ImageIntensifier)OptionA:GFD-200(DualRay-Q)(ClearedunderK172180)Activeimagearea:260x256mmCentralResolution:4.
6lp/mmType:CMOSResolution:2600x2560Pixelsamplingresolution:14bitsPixelpitch:100μmMTF:56%DQE:59%Scintillator:CsIOptionA:E5830SD-P4A(ClearedunderK140041)Activeimagearea:9"(9/6/4.
5)Min.
CentralResolutionatthemonitor:-9"(23cm):2.
2lp/mm-6"(15cm):2.
8lp/mm-6"(15cm):3.
0lp/mmDQE:65%(typically)E5830SD-P4AActiveimagearea:9"(9/6/4.
5)Min.
CentralResolutionatthemonitor:-9"(23cm):2.
2lp/mm-6"(15cm):2.
8lp/mm-6"(15cm):3.
0lp/mmDQE:65%(typically)OptionB:VIVIX-D0909GActiveimagearea:229.
12x229.
12mmCentralResolution:2.
7lp/mmType:a-SiTFTResolution:2600x2560Pixelsamplingresolution:16bitPixelpitch:179μmMTF:45%DQE:45%Scintillator:CsIOptionB:TH9438QX(ClearedunderK140041).
Activeimagearea:9"(9/6/4.
5)Min.
CentralResolutionatthemonitor:-9"(23cm):2.
2lp/mm-6"(15cm):2.
8lp/mm-6"(15cm):3.
0lp/mmDQE:65%(typically)Fluoroscopy40-120kV/0.
2-6.
0mA40-110kV/0.
2-6.
0mAPulsedFluoroscopy0.
2-10.
0mA0.
2-6.
0mARadiography40-120kV/0.
6-200mAs40-110kV/0.
4-100mAsDimensionsSID:1000mmSID:960mmPanningRotation:±12.
5PanningRotation:±12.
5OrbitalRotation:155OrbitalRotation:120Vert.
Travel:500mmVert.
Travel:400mmHoriz.
Travel:200mmHoriz.
Travel:200mmTheproposedOSCARisbasedonthepredicatedevice,ZEN-2090Pro(K091918).
ThereareseveraldifferencebetweenOSCAR(Prime,Classic)andpredicatedevice(ZEN-2090Pro)istheoptionsofImageacquisitionparts.
OSCARPrimeistheflatpaneldetectorandpredicatedeviceisImageIntensifier.
Asmentionedinthecomparisontable,predicatedeviceDQEishigherthanOSCARPrime.
However,predicatedeviceDQEistheDQEoftheimageintensifieritself.
Ingeneral,whenimageintensifieriscombinedwithCCDcameratheDQEdecreases.
Inconclusion,theDQEofthecompletepredicatedeviceis51%.
SotheDQEoftheOSCARPrimeismoreeffectiveandsafetythanpredicatedevice.
Alsoflatpaneldetectortypehasexcellentimageuniformity,nogeometricdistortion,noveilingglareorvignetting,smallandthinphysicalsizeascomparedtotheImageIntensifiertype.
TheOSCARissubstantiallyequivalenttothepredicatedevice,ZEN-2090Pro(K091918).
8.
Safety,EMCandPerformancedatacomparisontoPredicateOSCARcomplieswithindustrystandardssuchasIEC60601-1Seriesand21CFR1020.
30,21CFR1020.
31and21CFR1020.
32tominimizeelectrical,mechanicalandradiationhazards.
-Electrical,mechanical,environmentalsafetyandperformancetestingaccordingtostandardIEC60601-1,IEC60601-1-3,IEC60601-1-6,IEC60601-2-28,IEC60601-2-43,IEC60601-2-54andIEC62366wereperformed.
-EMCtestingwasconductedinaccordancewithstandardIEC60601-1-2.
-OSCARmeetstheEPRCstandards(21CFR1020.
30,31,32).
-FDAguidance"guidanceforSSXIdevices",and"guidancefortheContentofPremarketSubmissionsforSoftwareContainedinMedicaldevices",wasperformedforOSCAR.
Thesoftwarefeatureshasbeenchangedcomparetopredicatedeviceasbelow:EnhanceImageCalibrationfunction.
DisplayexposuremodesshowsontheLCDtouchpanel.
AddAngiographyfunctions.
Changestothepredicatedevicesoftwareweretestedandtheydonotaffectthedevicesafetyandeffectiveness.
Also,thedevicesoftwareismoderatelevelofconcern.
Asaresults,alltestresultsweresatisfactoryandtheresultofbenchandclinicalevaluationindicatesthatthenewdeviceisassafeandeffectiveasthepredicatedevice.
9.
ConclusionInreferencetothecomparisoninformationprovidedinsubstantialequivalencechart,andthemostoffunctionsandelectronicfeaturesaresimilarwithpredicatedevice.
WebelievethattheOSCARissafeandeffectiveaspredicatedevice,andhasnonewindicationforuse.
Therefore,OSCARissubstantiallyequivalenttopredicatedevice.
- practicesoray花生壳相关文档
- 地址oray花生壳
- 配置oray花生壳
- oray花生壳花生壳怎么用 花生壳动态域名使用方法
乌云数据(10/月),香港cera 1核1G 10M带宽/美国cera 8核8G10M
乌云数据主营高性价比国内外云服务器,物理机,本着机器为主服务为辅的运营理念,将客户的体验放在第一位,提供性价比最高的云服务器,帮助各位站长上云,同时我们深知新人站长的不易,特此提供永久免费虚拟主机,已提供两年之久,帮助了上万名站长从零上云官网:https://wuvps.cn迎国庆豪礼一多款机型史上最低价,续费不加价 尽在wuvps.cn香港cera机房,香港沙田机房,超低延迟CN2线路地区CPU...
10gbiz首月半价月付2.36美元,香港/洛杉矶VPS、硅谷独立服务器/站群服务器
收到10gbiz发来的7月份优惠方案,中国香港、美国洛杉矶机房VPS主机4折优惠码,优惠后洛杉矶VPS月付2.36美元起,香港VPS月付2.75美元起。这是一家2020年成立的主机商,提供的产品包括独立服务器租用和VPS主机等,数据中心在美国洛杉矶、圣何塞和中国香港。商家VPS主机基于KVM架构,支持使用PayPal或者支付宝付款。洛杉矶VPS架构CPU内存硬盘带宽系统价格单核512MB10GB1...
快云科技,免云服务器75折优惠服务器快云21元/月
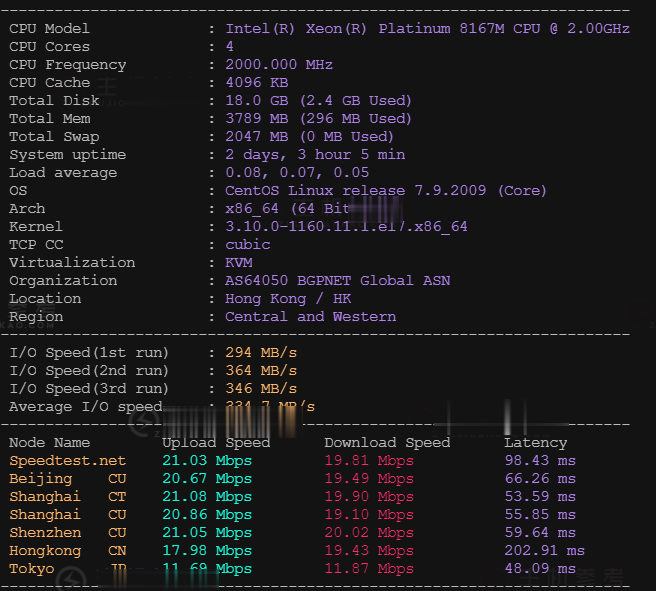
近日快云科技发布了最新的夏季优惠促销活动,主要针对旗下的香港CN2 GIA系列的VPS云服务器产品推送的最新的75折优惠码,国内回程三网CN2 GIA,平均延迟50ms以下,硬件配置方面采用E5 2696v2、E5 2696V4 铂金Platinum等,基于KVM虚拟架构,采用SSD硬盘存储,RAID10阵列保障数据安全,有需要香港免备案CN2服务器的朋友可以关注一下。快云科技怎么样?快云科技好不...
oray花生壳为你推荐
-
www.hao360.cn搜狗360导航网址是什么bbs.99nets.com怎么制作RO单机lunwenjiancewritecheck论文检测准吗?psbc.comwww.psbc.com怎样注册月神谭有没有什么好看的小说?拒绝言情小说!haole018.com为什么www.haole008.com在我这里打不开啊,是不是haole008换新的地址了?www.zjs.com.cn我的信用卡已经申请成功了,显示正在寄卡,怎么查询寄卡信息?www.se333se.com米奇网www.qvod333.com 看电影的效果好不?javbibibibi直播是真的吗www.123qqxx.com我的首页http://www.hao123.com被改成了http://www.669dh.cn/?yhc