Systemwww.6633.us
www.6633.us 时间:2021-04-07 阅读:()
2275ResearchBlvd.
,Ste.
300,Rockville,Maryland,USATelephone:+1-301-924-7077Fax:+1-301-924-7089Internete-mail:aoacri@aoac.
org*WorldWideWebSite:http://www.
aoac.
orgCERTIFICATIONAOACPerformanceTestedSMCertificateNo.
082003TheAOACResearchInstituteherebycertifiesthetestkitknownas:iQ-CheckEnterobacteriaceaemanufacturedbyBio-Radlaboratories2000AlfredNobelDr.
Hercules,CA94547USAThismethodhasbeenevaluatedintheAOACPerformanceTestedMethodsSMProgramandfoundtoperformasstatedbythemanufacturercontingenttothecommentscontainedinthemanuscript.
ThiscertificatemeansthatanAOACCertificationMarkLicenseAgreementhasbeenexecutedwhichauthorizesthemanufacturertodisplaytheAOACPerformanceTestedSMcertificationmarkalongwiththestatement-"THISMETHOD'SPERFORMANCEWASREVIEWEDBYAOACRESEARCHINSTITUTEANDWASFOUNDTOPERFORMTOTHEMANUFACTURER'SSPECIFICATIONS"-ontheabovementionedmethodforaperiodofonecalendaryearfromthedateofthiscertificateAugust18,2020–December31,2020).
Renewalmaybegrantedattheendofoneyearundertherulesstatedinthelicensingagreement.
August19,2020ScottCoates,SeniorDirectorSignatureforAOACResearchInstituteDateMETHODAUTHORSMikeClarkandWendyLauerSUBMITTINGCOMPANYBio-Radlaboratories2000AlfredNobelDr.
Hercules,CA94547USAKITNAME(S)iQ-CheckEnterobacteriaceaeCATALOGNUMBERS12003068INDEPENDENTLABORATORYWBAAnalyticalLaboratory3609JohnsonRd.
Springdale,AR72762USAAOACEXPERTSANDPEERREVIEWERSYiChen1,WayneZiemer2,JamesAgin31USFDACFSAN,CollegePark,MD,USA2Consultant,Loganville,GA,USA3QLaboratories,Inc.
,Cincinnati,OH45069APPLICABILITYOFMETHODAnalyte–Enterobacteriaceae(EB)Matrices–Milkpowder(10g),powderedinfantformula(10gand375g),powderedinfantformulawithprobiotics(10gand375g),andstainlesssteelenvironmentalswabsPerformanceclaims-TheiQ-CheckEnterobacteriaceaereal-timePCRkitisequivalenttothereferenceculturemethodISO21528-1:2017Microbiologyofthefoodchain–HorizontalmethodforthedetectionandenumerationofEnterobacteriaceae–Part1:DetectionofEnterobacteriaceae(2)forthefollowing:DetectionofEBfrom10gofmilkpowder,powderedinfantformula,andpowderedinfantformulawithprobioticsenrichedin90mlpre-warmedBPWand90mlofpre-warmedBPW+PIFSupplementfor18-22hat37±1°C.
DetectionofEBfrom375gofpowderedinfantformula,andpowderedinfantformulawithprobioticsenrichedin1,125mlofpre-warmedBPW+PIFSupplementfor18-22hat37±1°C.
DetectionofEBfroma4"x4"stainlesssurfaceswabshydratedwithD/ENeutralizingBrothandHiCapNeutralizingBrothenrichedin90mlofBPW18-22hat37±1°C.
ConfirmationofEBbystreakingtoRAPID'EnterobacteriaceaeagarfollowingenrichmentinBPWandBPW+PIFSupplement.
REFERENCEMETHODISO21528-1:2017Microbiologyofthefoodchain–HorizontalmethodforthedetectionandenumerationofEnterobacteriaceae–Part1:DetectionofEnterobacteriaceae.
(AccessedMay2020)https://sagaweb.
afnor.
org/fr-FR/sw/Consultation/Xml/1418735/lng=EN&supNumDos=XE023681(2)ORIGINALCERTIFICATIONDATEAugust18,2020CERTIFICATIONRENEWALRECORDNewApprovalMETHODMODIFICATIONRECORDNONESUMMARYOFMODIFICATIONNONEUnderthisAOACPerformanceTestedSMLicenseNumber,082003thismethodisdistributedby:NONEUnderthisAOACPerformanceTestedSMLicenseNumber,082003thismethodisdistributedas:NONEPRINCIPLEOFTHEMETHOD(1)TheiQ-CheckEnterobacteriaceaereal-timePCRassayisbasedongeneamplificationanddetection.
Thekit'sready-to-usePCRreagentscontainoligonucleotides(primersandprobes)specificforEB,aswellasDNApolymeraseandnucleotides.
DetectionanddataanalysisareoptimizedforusewithaBio-Radreal-timePCRinstrument,suchastheCFX96TouchDeepWellSystemwiththeCFXManagerIDEsoftware.
Inaddition,theiQ-CheckPrep,aroboticliquidhandlingplatformthatperformsDNAextractionandPCRplateset-up,canbeusedtoperformtheiQ-CheckEnterobacteriaceaereal-timePCRkit.
Thisallowsforacompletelyintegratedautomatedsolutionforfoodpathogentesting.
TheiQ-CheckFreeDNARemovalSolution,providedinaseparatekit,isusedwiththeiQ-CheckEnterobacteriaceaereal-timePCRkittooptimizeremovaloffreeDNA.
TheiQ-CheckEnterobacteriaceaereal-timePCRkitisprovidedinaready-to-useformatcontainingallprimers,probes,andreagents(exceptfortemplateDNA)requiredforthePCRreaction.
Inaddition,aninternalpositivecontrol(IPC)isincludedinthereactionmixtoidentifypossiblePCRinhibition.
Bio-RadiQ-CheckEnterobacteriaceae,AOACPerformanceTestedSMcertificationnumber082003DISCUSSIONOFTHEVALIDATIONSTUDY(2)TheiQ-CheckEnterobacteriaceaereal-timePCRkitsuccessfullydetectedEBfrom10gsampleportionsofmilkpowder,powderedinfantformula,andpowderedinfantformulawithprobioticsandstainlesssteelsurfacesincubatedwithBPW.
ThekitalsosuccessfullydetectedEB,from375gsampleportionsofpowderedinfantformula,andpowderedinfantformulawithprobioticsincubatedwithBPW+PIFSupplement.
UsingPODanalysis,nostatisticallysignificantdifferenceswereobservedbetweenthenumberofpositivesamplesdetectedbythecandidatemethodsandthereferencemethodsforallsamplestested.
ThestudyalsodemonstratedthatRAPID'EnterobacteriaceaeagarcouldbeusedasanalternativeconfirmationmediaafterenrichmentwhencomparedtothereferencemethodmediaofVRBG.
Intheinclusivityandexclusivityevaluations,allinclusivityorganismswerecorrectlyidentified,andallexclusivityorganismswerecorrectlyexcluded.
Thelot-to-lotconsistencyandstabilitystudyshownosignificantdifferencesobservedacrosstheshelflifeofthekitsforthreedifferentlotsofkitsateachtimepointtested.
UsingPODanalysis,therobustnessstudyshownostatisticallysignificantdifferencesbetweenthe8treatmentcombinationsandthenominalconditionfortheiQ-CheckEnterobacteriaceaekit.
TheiQ-CheckEnterobacteriaceaereal-timePCRmethodisquickandsimpletoperform,providingresultsinlessthanthreehourspostincubationoftheenrichmentforupto94samplereplicates.
TheuseoftheiQ-CheckPrepinstrumentcanprovideahands-freeapplicationthatcanreducepossiblecontaminationcausedbytheanalystperformingtesting.
TheiQ-CheckPrepinstrumentisabletoperformDNAextractionandPCRpreparationandprovidesaddedvalueoftraceabilitytothelab.
TheCFXManagerIDEsoftwareisuserfriendlywiththeabilitytotracklotinformationandsampleidentificationquicklyandwithease.
Sinceresultsaredisplayedinreal-time,theuserisabletoquicklyandaccuratelydetermineifresultswillbevalidbeforetheendoftherun.
ThesoftwarealsoprovidestheusertheoptiontoanalyzeeachindividualCqcurvestohelpaidinproblemsolvinganyissueswithinanindividualreaction.
Bio-RadiQ-CheckEnterobacteriaceae,AOACPerformanceTestedSMcertificationnumber082003Table3.
InclusivityResultsfortheiQ-CheckEnterobacteriaceaeAssay(1)No.
SpeciesSourceOriginBPWBPW+PIF1CitrobacteramalonaticusATCC125405Feces++2CitrobacterbraakiiATCC43162ClinicalIsolate++3CitrobacterfreundiiATCC43864Unknown++4CitrobacterfreundiiATCC8090Unknown++5CitrobacterkoseriATCC27156CDC++6CronobactersakazakiiATCC29544Infantformula++7CronobactersakazakiiATCC12868Unknown++8CronobactersakazakiiATCC29004Unknown++9EdwardsiellatardaDSMZ230052Unknown++10EnterobacteraerogenesATCC13048Unknown++11EnterobacteramnigenusATCC33072Soil++12EnterobacterasburiaeATCC35953Unknown++13EnterobactercloacaeATCC35030Unknown++14EnterobactercloacaeATCC13047Spinalfluid++15EnterobactergergoviaeDSMZ9245Unknown++16EnterobacterhormaecheiATCC49162Sputum++17EscherichiacoliATCC11775Unknown++18EscherichiacoliO26ATCCBAA-1653Unknown++19EscherichiacoliO157:H7ATCC35150HumanFeces++20EscherichiafergusoniiATCC35469Humanfeces++21EscherichiahermaniiATCC33650Mousebrain++22EscherichiavulnerisATCC29943Humanwound++23FranconibacterpulverisDSMZ19144Unknown++24HafniaalveiATCC51815Milk++25HafniaalveiATCC29926Human++26KlebsiellaoxytocaATCC43165Clinicalisolate++27KlebsiellapneaumoniaeATCC4352CowMilk++28KluyveracryocrescensDSMZ4588Unknown++29MorganellamorganiiATCC25829Human++30PantoeaagglomeransATCC19552Sewage++31PantoeadispersaDSMZ30073Unknown++32ProteusmirabilisATCC29906Unknown++33ProteusvulgarisDSMZ13387Unknown++34ProteusvulgarisATCC21719Unknown++35RahnellaaquatilisATCC55046Soil++36RaoultellaterrigenaDSMZ2687Unknown++37SalmonellaAbaetetubaATCC35640Creekwater++38SalmonellaHadarATCC51956Unknown++39SalmonellaKahlaATCC17980Unknown++40SalmonellaKentuckyATCC9263Unknown++41SalmonellaPulorumATCC13036Egg++42SalmonellaSenftenbergATCC43845Unknown++43SalmonellaTyphimuriumATCC29946Unknown++44SerratiaficariaATCC33106Figtree++Bio-RadiQ-CheckEnterobacteriaceae,AOACPerformanceTestedSMcertificationnumber08200345SerratiamarcescensATCC13880Human++46SerratiamarcescensATCC27137Human++47SerratiaproteamaculansATCC35474Unknown++48ShigellaflexneriDSMZ4782Unknown++49ShigellasonneiDSMZ5570Unknown++50YersiniaaldovaeATCC51366Water++51YersiniaenterocoliticaATCC9610HumanTissue++1AmericanTypeCultureCollection,Manassas,VA2TheLeibnizInstituteDSMZ,Brunswick,GermanyTable4.
ExclusivityResultsfortheiQ-CheckEnterobacteriaceaeAssay(1)No.
SpeciesSourceOriginResult1AcinetobacterbaumanniiDSMZ130007Urine-2AcinetobactercalcoaceticusATCC223055Unknown-3AeromonashydrophiliaDSMZ30187Milk-4AeromonasmediaDSMZ4881Fish-5AeromonassobriaDSMZ19176Fish-6AlcaligenesfaecalisDSMZ30030Unknown-7BacillusmegateriumATCC12872Unknown-8BacillussubtilusATCC6633Unknown-9BurkholderiacepaciaDSMZ7288Onion-10BurkholderiacontaminansDSMZ22706Sheepmilk-11StaphylococcusepidermidisATCC12228Unknown-12EnterococcusfaecalisDSMZ20478Unknown-13LactobacilluslactisATCC11454Unknown-14LeuconostocmesenteroidesATCC8293Fermentingolives-15ListeriamonocytogenesATCC19114Animaltissue-16MicrococcusluteusATCC9341Soil-17MoraxellacatarrhalisDSMZ9143Unknown-18PasteurellaaerogenesDSMZ10153Pig-19PseudomonasfluorescensDSMZ50091Water-20PseudomonasaeruginosaATCC10145Unknown-21PseudomonasputidaDSMZ291Unknown-22RhodococcusequiATCC6939Foal-23RothiamucilaginosaDSMZ20746Humanthroat-24StaphylococcusaureusATCC6538Human-25StenotrophomonasacidaminiphilaDSMZ13117Wastewater-26StenotrophomonasmaltophiliaDSMZ50170Human-27StreptococcusagalactiaeATCCBAA-611Human-28StreptococcusfaecalisATCC29212Urine-29FlavobacteriumbranchiophilumDSMZ24789Unknown-30MoraxellabovisDSMZ6328Cow-1TheLeibnizInstituteDSMZ,Brunswick,Germany2AmericanTypeCultureCollection,Manassas,VATable6:Candidatevs.
ISO21528-1:2017ReferenceMethod–PODResults(1)MatrixStrainEnrichmentMPNaTestPortionNbCandidateReferencedPODCf95%CIgXcPODCd95%CIXPODRe95%CIMilkPowder(10g)S.
AnatumUSMARC1735BPWh(Paired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
470.
56(0.
32,0.
98)2070.
350.
18,0.
5780.
400.
22,0.
61-0.
05-0.
32,0.
231.
63(0.
77,3.
44)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47MilkPowder(10g)S.
AnatumUSMARC1735BPW+PIFi(Unpaired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
43,0.
430.
56(0.
32,0.
98)2090.
450.
26,0.
6680.
400.
22,0.
610.
05-0.
24,0.
331.
63(0.
77,3.
44)530.
600.
23,0.
8851.
000.
57,1.
00-0.
40-0.
77,0.
12Powderedinfantformula(10g)E.
coliATCC25922BPW(Paired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
17(0.
74,1.
86)20140.
700.
48,0.
85140.
700.
48,0.
850.
00-0.
27,0.
275.
24(2.
40,11.
48)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformula(10g)E.
coliATCC25922BPW+PIF(Unpaired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
43,0.
431.
17(0.
74,1.
86)2090.
450.
26,0.
66140.
700.
48,0.
85-0.
25-0.
50,0.
055.
24(2.
40,11.
48)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
43,0.
43Powderedinfantformula(375g)E.
coliATCC25922BPW+PIF(Unpaired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
43,0.
431.
17(0.
74,1.
86)20160.
800.
58,0.
92140.
700.
48,0.
850.
10-0.
17,0.
355.
24(2.
40,11.
48)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
43,0.
43Powderedinfantformulaw/probiotics(10g)C.
sakazakiiATCC29544BPW(Paired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
65(1.
04,2.
62)20150.
750.
53,0.
89150.
750.
53,0.
890.
00-0.
26,0.
267.
43(3.
08,17.
94)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformulaw/probiotics(10g)C.
sakazakiiATCC29544BPW+PIF(Unpaired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
43,0.
431.
65(1.
04,2.
62)20170.
850.
64,0.
95150.
750.
53,0.
890.
10-0.
15,0.
347.
43(3.
08,17.
94)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
43,0.
43Powderedinfantformulaw/probiotics(375g)C.
sakazakiiATCC29544BPW+PIF(Unpaired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
43,0.
431.
65(1.
04,2.
62)20190.
950.
76,1.
00150.
750.
53,0.
890.
20-0.
03,0.
427.
43(3.
08,17.
94)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
43,0.
43aMPN=MostProbableNumberiscalculatedusingtheFDABAM,with95%confidenceintervalbN=Numberoftestportionscx=NumberofpositivetestportionsdPODC=CandidatemethodconfirmedpositiveoutcomesdividedbythetotalnumberoftrialsBio-RadiQ-CheckEnterobacteriaceae,AOACPerformanceTestedSMcertificationnumber082003ePODR=ReferencemethodconfirmedpositiveoutcomesdividedbythetotalnumberoftrialsfdPODC=DifferencebetweentheconfirmedcandidatemethodresultandreferencemethodconfirmedresultPODvaluesg95%CI=IftheconfidenceintervalofadPODdoesnotcontainzero,thenthedifferenceisstatisticallysignificantatthe5%levelhBPW=BufferedPeptoneWateriBPW+PIF=BufferedPeptoneWater+PIFSupplementTable7:Candidatevs.
ISO21528-1:2017ReferenceMethod–PODResults(1)MatrixStrainEnrichmentCFUaTestPortionNbCandidateReferencedPODCf95%CIgXcPODCd95%CIXPODRe95%CIStainlessSteel(4"x4")Cellulose+D/EK.
aerogenesATCC13048+P.
aeruginosaATCC9027BPWh(Paired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
473.
5x102&4.
0x10320120.
600.
39,0.
78120.
600.
39,0.
780.
00-0.
13,0.
131.
7x104&1.
8x105551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47StainlessSteel(4"x4")Polyurethane+HiCapK.
aerogenesATCC13048+P.
aeruginosaATCC9027BPW(Paired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
473.
5x102&4.
0x1032090.
450.
26,0.
6690.
450.
26,0.
660.
00-0.
13,0.
131.
7x104&1.
8x105551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47aCFU=ResultsoftheCFU/TestareaweredeterminedbyplatingtheinoculumforeachsurfaceintriplicatebN=Numberoftestportionscx=NumberofpositivetestportionsdPODC=CandidatemethodconfirmedpositiveoutcomesdividedbythetotalnumberoftrialsePODR=ReferencemethodconfirmedpositiveoutcomesdividedbythetotalnumberoftrialsfdPODC=DifferencebetweentheconfirmedcandidatemethodresultandreferencemethodconfirmedresultPODvaluesg95%CI=IftheconfidenceintervalofadPODdoesnotcontainzero,thenthedifferenceisstatisticallysignificantatthe5%levelhBPW=BufferedPeptoneWaterTable8:iQ-CheckEnterobacteriaceaePresumptivevs.
Confirmed–PODResults(1)MatrixStrainEnrichmentMPNaTestPortionNbPresumptiveConfirmeddPODCPf95%CIgXcPODCPd95%CIXPODCCe95%CIMilkPowder(10g)S.
AnatumUSMARC1735BPWh-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
470.
56(0.
32,0.
98)2070.
350.
18,0.
5780.
400.
22,0.
61-0.
05-0.
21,0.
111.
63(0.
77,3.
44)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47MilkPowder(10g)S.
AnatumUSMARC1735BPW+PIFi-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
470.
56(0.
32,0.
98)2090.
450.
26,0.
66100.
500.
30,0.
70-0.
05-0.
21,0.
111.
63(0.
77,3.
44)530.
600.
23,0.
8830.
600.
23,0.
880.
00-0.
47,0.
47PowderedE.
coliBPW-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
47Bio-RadiQ-CheckEnterobacteriaceae,AOACPerformanceTestedSMcertificationnumber082003infantformula(10g)ATCC259221.
17(0.
74,1.
86)20140.
700.
48,0.
85140.
700.
48,0.
850.
00-0.
13,0.
135.
24(2.
40,11.
48)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformula(10g)E.
coliATCC25922BPW+PIF-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
17(0.
74,1.
86)2090.
450.
26,0.
6690.
450.
26,0.
660.
00-0.
13,0.
135.
24(2.
40,11.
48)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformula(375g)E.
coliATCC25922BPW+PIF-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
17(0.
74,1.
86)20160.
800.
58,0.
92160.
800.
58,0.
920.
00-0.
13,0.
135.
24(2.
40,11.
48)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformulaw/probiotics(10g)C.
sakazakiiATCC29544BPW-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
65(1.
04,2.
62)20150.
750.
53,0.
89150.
750.
53,0.
890.
00-0.
13,0.
137.
43(3.
08,17.
94)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformulaw/probiotics(10g)C.
sakazakiiATCC29544BPW+PIF-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
65(1.
04,2.
62)20170.
850.
64,0.
95170.
850.
64,0.
950.
00-0.
13,0.
137.
43(3.
08,17.
94)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformulaw/probiotics(375g)C.
sakazakiiATCC29544BPW+PIF-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
65(1.
04,2.
62)20201.
000.
84,1.
00190.
950.
76,1.
000.
05-0.
11,0.
217.
43(3.
08,17.
94)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47aMPN=MostProbableNumberiscalculatedusingtheFDABAM,with95%confidenceintervalbN=Numberoftestportionscx=NumberofpositivetestportionsdPODCP=CandidatemethodpresumptivepositiveoutcomesdividedbythetotalnumberoftrialsePODCC=CandidatemethodconfirmedpositiveoutcomesdividedbythetotalnumberoftrialsfdPODCP=DifferencebetweenthepresumptiveandconfirmedcandidatemethodresultPODvaluesg95%CI=IftheconfidenceintervalofadPODdoesnotcontainzero,thenthedifferenceisstatisticallysignificantatthe5%levelhBPW=BufferedPeptoneWateriBPW+PIF=BufferedPeptoneWater+PIFSupplementBio-RadiQ-CheckEnterobacteriaceae,AOACPerformanceTestedSMcertificationnumber082003Table9:iQ-CheckEnterobacteriaceaePresumptivevs.
Confirmed–PODResults(1)MatrixStrainEnrichmentCFUaTestPortionNbPresumptiveConfirmeddPODCPf95%CIgXcPODCPd95%CIXPODCCe95%CIStainlessSteel(4"x4")Cellulose+D/EK.
aerogenesATCC13048+P.
aeruginosaATCC9027BPWh-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
473.
5x102&4.
0x10320120.
600.
39,0.
78120.
600.
39,0.
780.
00-0.
13,0.
131.
7x104&1.
8x105551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47StainlessSteel(4"x4")Polyurethane+HiCapK.
aerogenesATCC13048+P.
aeruginosaATCC9027BPW-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
473.
5x102&4.
0x1032090.
450.
26,0.
6690.
450.
26,0.
660.
00-0.
13,0.
131.
7x104&1.
8x105551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47aCFU=ResultsoftheCFU/TestareaweredeterminedbyplatingtheinoculumforeachsurfaceintriplicatebN=Numberoftestportionscx=NumberofpositivetestportionsdPODCP=CandidatemethodpresumptivepositiveoutcomesdividedbythetotalnumberoftrialsePODCC=CandidatemethodconfirmedpositiveoutcomesdividedbythetotalnumberoftrialsfdPODCP=DifferencebetweenthepresumptiveandconfirmedcandidatemethodresultPODvaluesg95%CI=IftheconfidenceintervalofadPODdoesnotcontainzero,thenthedifferenceisstatisticallysignificantatthe5%levelhBPW=BufferedPeptoneWaterREFERENCESCITED(1)Clark,M.
andLauer,W.
,ValidationoftheiQ-CheckEnterobacteriaceaeReal-TimePCRMethodfortheDetectionofEnterobacteriaceaeinSelectMatrixesandEnvironmentalSurfaces,AOACPerformanceTestedSMcertificationnumber082003.
(2)ISO21528-1:2017Microbiologyofthefoodchain–HorizontalmethodforthedetectionandenumerationofEnterobacteriaceae–Part1:DetectionofEnterobacteriaceae.
(AccessedMay2020)https://sagaweb.
afnor.
org/fr-FR/sw/Consultation/Xml/1418735/lng=EN&supNumDos=XE023681
,Ste.
300,Rockville,Maryland,USATelephone:+1-301-924-7077Fax:+1-301-924-7089Internete-mail:aoacri@aoac.
org*WorldWideWebSite:http://www.
aoac.
orgCERTIFICATIONAOACPerformanceTestedSMCertificateNo.
082003TheAOACResearchInstituteherebycertifiesthetestkitknownas:iQ-CheckEnterobacteriaceaemanufacturedbyBio-Radlaboratories2000AlfredNobelDr.
Hercules,CA94547USAThismethodhasbeenevaluatedintheAOACPerformanceTestedMethodsSMProgramandfoundtoperformasstatedbythemanufacturercontingenttothecommentscontainedinthemanuscript.
ThiscertificatemeansthatanAOACCertificationMarkLicenseAgreementhasbeenexecutedwhichauthorizesthemanufacturertodisplaytheAOACPerformanceTestedSMcertificationmarkalongwiththestatement-"THISMETHOD'SPERFORMANCEWASREVIEWEDBYAOACRESEARCHINSTITUTEANDWASFOUNDTOPERFORMTOTHEMANUFACTURER'SSPECIFICATIONS"-ontheabovementionedmethodforaperiodofonecalendaryearfromthedateofthiscertificateAugust18,2020–December31,2020).
Renewalmaybegrantedattheendofoneyearundertherulesstatedinthelicensingagreement.
August19,2020ScottCoates,SeniorDirectorSignatureforAOACResearchInstituteDateMETHODAUTHORSMikeClarkandWendyLauerSUBMITTINGCOMPANYBio-Radlaboratories2000AlfredNobelDr.
Hercules,CA94547USAKITNAME(S)iQ-CheckEnterobacteriaceaeCATALOGNUMBERS12003068INDEPENDENTLABORATORYWBAAnalyticalLaboratory3609JohnsonRd.
Springdale,AR72762USAAOACEXPERTSANDPEERREVIEWERSYiChen1,WayneZiemer2,JamesAgin31USFDACFSAN,CollegePark,MD,USA2Consultant,Loganville,GA,USA3QLaboratories,Inc.
,Cincinnati,OH45069APPLICABILITYOFMETHODAnalyte–Enterobacteriaceae(EB)Matrices–Milkpowder(10g),powderedinfantformula(10gand375g),powderedinfantformulawithprobiotics(10gand375g),andstainlesssteelenvironmentalswabsPerformanceclaims-TheiQ-CheckEnterobacteriaceaereal-timePCRkitisequivalenttothereferenceculturemethodISO21528-1:2017Microbiologyofthefoodchain–HorizontalmethodforthedetectionandenumerationofEnterobacteriaceae–Part1:DetectionofEnterobacteriaceae(2)forthefollowing:DetectionofEBfrom10gofmilkpowder,powderedinfantformula,andpowderedinfantformulawithprobioticsenrichedin90mlpre-warmedBPWand90mlofpre-warmedBPW+PIFSupplementfor18-22hat37±1°C.
DetectionofEBfrom375gofpowderedinfantformula,andpowderedinfantformulawithprobioticsenrichedin1,125mlofpre-warmedBPW+PIFSupplementfor18-22hat37±1°C.
DetectionofEBfroma4"x4"stainlesssurfaceswabshydratedwithD/ENeutralizingBrothandHiCapNeutralizingBrothenrichedin90mlofBPW18-22hat37±1°C.
ConfirmationofEBbystreakingtoRAPID'EnterobacteriaceaeagarfollowingenrichmentinBPWandBPW+PIFSupplement.
REFERENCEMETHODISO21528-1:2017Microbiologyofthefoodchain–HorizontalmethodforthedetectionandenumerationofEnterobacteriaceae–Part1:DetectionofEnterobacteriaceae.
(AccessedMay2020)https://sagaweb.
afnor.
org/fr-FR/sw/Consultation/Xml/1418735/lng=EN&supNumDos=XE023681(2)ORIGINALCERTIFICATIONDATEAugust18,2020CERTIFICATIONRENEWALRECORDNewApprovalMETHODMODIFICATIONRECORDNONESUMMARYOFMODIFICATIONNONEUnderthisAOACPerformanceTestedSMLicenseNumber,082003thismethodisdistributedby:NONEUnderthisAOACPerformanceTestedSMLicenseNumber,082003thismethodisdistributedas:NONEPRINCIPLEOFTHEMETHOD(1)TheiQ-CheckEnterobacteriaceaereal-timePCRassayisbasedongeneamplificationanddetection.
Thekit'sready-to-usePCRreagentscontainoligonucleotides(primersandprobes)specificforEB,aswellasDNApolymeraseandnucleotides.
DetectionanddataanalysisareoptimizedforusewithaBio-Radreal-timePCRinstrument,suchastheCFX96TouchDeepWellSystemwiththeCFXManagerIDEsoftware.
Inaddition,theiQ-CheckPrep,aroboticliquidhandlingplatformthatperformsDNAextractionandPCRplateset-up,canbeusedtoperformtheiQ-CheckEnterobacteriaceaereal-timePCRkit.
Thisallowsforacompletelyintegratedautomatedsolutionforfoodpathogentesting.
TheiQ-CheckFreeDNARemovalSolution,providedinaseparatekit,isusedwiththeiQ-CheckEnterobacteriaceaereal-timePCRkittooptimizeremovaloffreeDNA.
TheiQ-CheckEnterobacteriaceaereal-timePCRkitisprovidedinaready-to-useformatcontainingallprimers,probes,andreagents(exceptfortemplateDNA)requiredforthePCRreaction.
Inaddition,aninternalpositivecontrol(IPC)isincludedinthereactionmixtoidentifypossiblePCRinhibition.
Bio-RadiQ-CheckEnterobacteriaceae,AOACPerformanceTestedSMcertificationnumber082003DISCUSSIONOFTHEVALIDATIONSTUDY(2)TheiQ-CheckEnterobacteriaceaereal-timePCRkitsuccessfullydetectedEBfrom10gsampleportionsofmilkpowder,powderedinfantformula,andpowderedinfantformulawithprobioticsandstainlesssteelsurfacesincubatedwithBPW.
ThekitalsosuccessfullydetectedEB,from375gsampleportionsofpowderedinfantformula,andpowderedinfantformulawithprobioticsincubatedwithBPW+PIFSupplement.
UsingPODanalysis,nostatisticallysignificantdifferenceswereobservedbetweenthenumberofpositivesamplesdetectedbythecandidatemethodsandthereferencemethodsforallsamplestested.
ThestudyalsodemonstratedthatRAPID'EnterobacteriaceaeagarcouldbeusedasanalternativeconfirmationmediaafterenrichmentwhencomparedtothereferencemethodmediaofVRBG.
Intheinclusivityandexclusivityevaluations,allinclusivityorganismswerecorrectlyidentified,andallexclusivityorganismswerecorrectlyexcluded.
Thelot-to-lotconsistencyandstabilitystudyshownosignificantdifferencesobservedacrosstheshelflifeofthekitsforthreedifferentlotsofkitsateachtimepointtested.
UsingPODanalysis,therobustnessstudyshownostatisticallysignificantdifferencesbetweenthe8treatmentcombinationsandthenominalconditionfortheiQ-CheckEnterobacteriaceaekit.
TheiQ-CheckEnterobacteriaceaereal-timePCRmethodisquickandsimpletoperform,providingresultsinlessthanthreehourspostincubationoftheenrichmentforupto94samplereplicates.
TheuseoftheiQ-CheckPrepinstrumentcanprovideahands-freeapplicationthatcanreducepossiblecontaminationcausedbytheanalystperformingtesting.
TheiQ-CheckPrepinstrumentisabletoperformDNAextractionandPCRpreparationandprovidesaddedvalueoftraceabilitytothelab.
TheCFXManagerIDEsoftwareisuserfriendlywiththeabilitytotracklotinformationandsampleidentificationquicklyandwithease.
Sinceresultsaredisplayedinreal-time,theuserisabletoquicklyandaccuratelydetermineifresultswillbevalidbeforetheendoftherun.
ThesoftwarealsoprovidestheusertheoptiontoanalyzeeachindividualCqcurvestohelpaidinproblemsolvinganyissueswithinanindividualreaction.
Bio-RadiQ-CheckEnterobacteriaceae,AOACPerformanceTestedSMcertificationnumber082003Table3.
InclusivityResultsfortheiQ-CheckEnterobacteriaceaeAssay(1)No.
SpeciesSourceOriginBPWBPW+PIF1CitrobacteramalonaticusATCC125405Feces++2CitrobacterbraakiiATCC43162ClinicalIsolate++3CitrobacterfreundiiATCC43864Unknown++4CitrobacterfreundiiATCC8090Unknown++5CitrobacterkoseriATCC27156CDC++6CronobactersakazakiiATCC29544Infantformula++7CronobactersakazakiiATCC12868Unknown++8CronobactersakazakiiATCC29004Unknown++9EdwardsiellatardaDSMZ230052Unknown++10EnterobacteraerogenesATCC13048Unknown++11EnterobacteramnigenusATCC33072Soil++12EnterobacterasburiaeATCC35953Unknown++13EnterobactercloacaeATCC35030Unknown++14EnterobactercloacaeATCC13047Spinalfluid++15EnterobactergergoviaeDSMZ9245Unknown++16EnterobacterhormaecheiATCC49162Sputum++17EscherichiacoliATCC11775Unknown++18EscherichiacoliO26ATCCBAA-1653Unknown++19EscherichiacoliO157:H7ATCC35150HumanFeces++20EscherichiafergusoniiATCC35469Humanfeces++21EscherichiahermaniiATCC33650Mousebrain++22EscherichiavulnerisATCC29943Humanwound++23FranconibacterpulverisDSMZ19144Unknown++24HafniaalveiATCC51815Milk++25HafniaalveiATCC29926Human++26KlebsiellaoxytocaATCC43165Clinicalisolate++27KlebsiellapneaumoniaeATCC4352CowMilk++28KluyveracryocrescensDSMZ4588Unknown++29MorganellamorganiiATCC25829Human++30PantoeaagglomeransATCC19552Sewage++31PantoeadispersaDSMZ30073Unknown++32ProteusmirabilisATCC29906Unknown++33ProteusvulgarisDSMZ13387Unknown++34ProteusvulgarisATCC21719Unknown++35RahnellaaquatilisATCC55046Soil++36RaoultellaterrigenaDSMZ2687Unknown++37SalmonellaAbaetetubaATCC35640Creekwater++38SalmonellaHadarATCC51956Unknown++39SalmonellaKahlaATCC17980Unknown++40SalmonellaKentuckyATCC9263Unknown++41SalmonellaPulorumATCC13036Egg++42SalmonellaSenftenbergATCC43845Unknown++43SalmonellaTyphimuriumATCC29946Unknown++44SerratiaficariaATCC33106Figtree++Bio-RadiQ-CheckEnterobacteriaceae,AOACPerformanceTestedSMcertificationnumber08200345SerratiamarcescensATCC13880Human++46SerratiamarcescensATCC27137Human++47SerratiaproteamaculansATCC35474Unknown++48ShigellaflexneriDSMZ4782Unknown++49ShigellasonneiDSMZ5570Unknown++50YersiniaaldovaeATCC51366Water++51YersiniaenterocoliticaATCC9610HumanTissue++1AmericanTypeCultureCollection,Manassas,VA2TheLeibnizInstituteDSMZ,Brunswick,GermanyTable4.
ExclusivityResultsfortheiQ-CheckEnterobacteriaceaeAssay(1)No.
SpeciesSourceOriginResult1AcinetobacterbaumanniiDSMZ130007Urine-2AcinetobactercalcoaceticusATCC223055Unknown-3AeromonashydrophiliaDSMZ30187Milk-4AeromonasmediaDSMZ4881Fish-5AeromonassobriaDSMZ19176Fish-6AlcaligenesfaecalisDSMZ30030Unknown-7BacillusmegateriumATCC12872Unknown-8BacillussubtilusATCC6633Unknown-9BurkholderiacepaciaDSMZ7288Onion-10BurkholderiacontaminansDSMZ22706Sheepmilk-11StaphylococcusepidermidisATCC12228Unknown-12EnterococcusfaecalisDSMZ20478Unknown-13LactobacilluslactisATCC11454Unknown-14LeuconostocmesenteroidesATCC8293Fermentingolives-15ListeriamonocytogenesATCC19114Animaltissue-16MicrococcusluteusATCC9341Soil-17MoraxellacatarrhalisDSMZ9143Unknown-18PasteurellaaerogenesDSMZ10153Pig-19PseudomonasfluorescensDSMZ50091Water-20PseudomonasaeruginosaATCC10145Unknown-21PseudomonasputidaDSMZ291Unknown-22RhodococcusequiATCC6939Foal-23RothiamucilaginosaDSMZ20746Humanthroat-24StaphylococcusaureusATCC6538Human-25StenotrophomonasacidaminiphilaDSMZ13117Wastewater-26StenotrophomonasmaltophiliaDSMZ50170Human-27StreptococcusagalactiaeATCCBAA-611Human-28StreptococcusfaecalisATCC29212Urine-29FlavobacteriumbranchiophilumDSMZ24789Unknown-30MoraxellabovisDSMZ6328Cow-1TheLeibnizInstituteDSMZ,Brunswick,Germany2AmericanTypeCultureCollection,Manassas,VATable6:Candidatevs.
ISO21528-1:2017ReferenceMethod–PODResults(1)MatrixStrainEnrichmentMPNaTestPortionNbCandidateReferencedPODCf95%CIgXcPODCd95%CIXPODRe95%CIMilkPowder(10g)S.
AnatumUSMARC1735BPWh(Paired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
470.
56(0.
32,0.
98)2070.
350.
18,0.
5780.
400.
22,0.
61-0.
05-0.
32,0.
231.
63(0.
77,3.
44)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47MilkPowder(10g)S.
AnatumUSMARC1735BPW+PIFi(Unpaired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
43,0.
430.
56(0.
32,0.
98)2090.
450.
26,0.
6680.
400.
22,0.
610.
05-0.
24,0.
331.
63(0.
77,3.
44)530.
600.
23,0.
8851.
000.
57,1.
00-0.
40-0.
77,0.
12Powderedinfantformula(10g)E.
coliATCC25922BPW(Paired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
17(0.
74,1.
86)20140.
700.
48,0.
85140.
700.
48,0.
850.
00-0.
27,0.
275.
24(2.
40,11.
48)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformula(10g)E.
coliATCC25922BPW+PIF(Unpaired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
43,0.
431.
17(0.
74,1.
86)2090.
450.
26,0.
66140.
700.
48,0.
85-0.
25-0.
50,0.
055.
24(2.
40,11.
48)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
43,0.
43Powderedinfantformula(375g)E.
coliATCC25922BPW+PIF(Unpaired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
43,0.
431.
17(0.
74,1.
86)20160.
800.
58,0.
92140.
700.
48,0.
850.
10-0.
17,0.
355.
24(2.
40,11.
48)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
43,0.
43Powderedinfantformulaw/probiotics(10g)C.
sakazakiiATCC29544BPW(Paired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
65(1.
04,2.
62)20150.
750.
53,0.
89150.
750.
53,0.
890.
00-0.
26,0.
267.
43(3.
08,17.
94)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformulaw/probiotics(10g)C.
sakazakiiATCC29544BPW+PIF(Unpaired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
43,0.
431.
65(1.
04,2.
62)20170.
850.
64,0.
95150.
750.
53,0.
890.
10-0.
15,0.
347.
43(3.
08,17.
94)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
43,0.
43Powderedinfantformulaw/probiotics(375g)C.
sakazakiiATCC29544BPW+PIF(Unpaired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
43,0.
431.
65(1.
04,2.
62)20190.
950.
76,1.
00150.
750.
53,0.
890.
20-0.
03,0.
427.
43(3.
08,17.
94)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
43,0.
43aMPN=MostProbableNumberiscalculatedusingtheFDABAM,with95%confidenceintervalbN=Numberoftestportionscx=NumberofpositivetestportionsdPODC=CandidatemethodconfirmedpositiveoutcomesdividedbythetotalnumberoftrialsBio-RadiQ-CheckEnterobacteriaceae,AOACPerformanceTestedSMcertificationnumber082003ePODR=ReferencemethodconfirmedpositiveoutcomesdividedbythetotalnumberoftrialsfdPODC=DifferencebetweentheconfirmedcandidatemethodresultandreferencemethodconfirmedresultPODvaluesg95%CI=IftheconfidenceintervalofadPODdoesnotcontainzero,thenthedifferenceisstatisticallysignificantatthe5%levelhBPW=BufferedPeptoneWateriBPW+PIF=BufferedPeptoneWater+PIFSupplementTable7:Candidatevs.
ISO21528-1:2017ReferenceMethod–PODResults(1)MatrixStrainEnrichmentCFUaTestPortionNbCandidateReferencedPODCf95%CIgXcPODCd95%CIXPODRe95%CIStainlessSteel(4"x4")Cellulose+D/EK.
aerogenesATCC13048+P.
aeruginosaATCC9027BPWh(Paired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
473.
5x102&4.
0x10320120.
600.
39,0.
78120.
600.
39,0.
780.
00-0.
13,0.
131.
7x104&1.
8x105551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47StainlessSteel(4"x4")Polyurethane+HiCapK.
aerogenesATCC13048+P.
aeruginosaATCC9027BPW(Paired)-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
473.
5x102&4.
0x1032090.
450.
26,0.
6690.
450.
26,0.
660.
00-0.
13,0.
131.
7x104&1.
8x105551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47aCFU=ResultsoftheCFU/TestareaweredeterminedbyplatingtheinoculumforeachsurfaceintriplicatebN=Numberoftestportionscx=NumberofpositivetestportionsdPODC=CandidatemethodconfirmedpositiveoutcomesdividedbythetotalnumberoftrialsePODR=ReferencemethodconfirmedpositiveoutcomesdividedbythetotalnumberoftrialsfdPODC=DifferencebetweentheconfirmedcandidatemethodresultandreferencemethodconfirmedresultPODvaluesg95%CI=IftheconfidenceintervalofadPODdoesnotcontainzero,thenthedifferenceisstatisticallysignificantatthe5%levelhBPW=BufferedPeptoneWaterTable8:iQ-CheckEnterobacteriaceaePresumptivevs.
Confirmed–PODResults(1)MatrixStrainEnrichmentMPNaTestPortionNbPresumptiveConfirmeddPODCPf95%CIgXcPODCPd95%CIXPODCCe95%CIMilkPowder(10g)S.
AnatumUSMARC1735BPWh-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
470.
56(0.
32,0.
98)2070.
350.
18,0.
5780.
400.
22,0.
61-0.
05-0.
21,0.
111.
63(0.
77,3.
44)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47MilkPowder(10g)S.
AnatumUSMARC1735BPW+PIFi-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
470.
56(0.
32,0.
98)2090.
450.
26,0.
66100.
500.
30,0.
70-0.
05-0.
21,0.
111.
63(0.
77,3.
44)530.
600.
23,0.
8830.
600.
23,0.
880.
00-0.
47,0.
47PowderedE.
coliBPW-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
47Bio-RadiQ-CheckEnterobacteriaceae,AOACPerformanceTestedSMcertificationnumber082003infantformula(10g)ATCC259221.
17(0.
74,1.
86)20140.
700.
48,0.
85140.
700.
48,0.
850.
00-0.
13,0.
135.
24(2.
40,11.
48)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformula(10g)E.
coliATCC25922BPW+PIF-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
17(0.
74,1.
86)2090.
450.
26,0.
6690.
450.
26,0.
660.
00-0.
13,0.
135.
24(2.
40,11.
48)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformula(375g)E.
coliATCC25922BPW+PIF-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
17(0.
74,1.
86)20160.
800.
58,0.
92160.
800.
58,0.
920.
00-0.
13,0.
135.
24(2.
40,11.
48)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformulaw/probiotics(10g)C.
sakazakiiATCC29544BPW-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
65(1.
04,2.
62)20150.
750.
53,0.
89150.
750.
53,0.
890.
00-0.
13,0.
137.
43(3.
08,17.
94)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformulaw/probiotics(10g)C.
sakazakiiATCC29544BPW+PIF-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
65(1.
04,2.
62)20170.
850.
64,0.
95170.
850.
64,0.
950.
00-0.
13,0.
137.
43(3.
08,17.
94)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47Powderedinfantformulaw/probiotics(375g)C.
sakazakiiATCC29544BPW+PIF-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
471.
65(1.
04,2.
62)20201.
000.
84,1.
00190.
950.
76,1.
000.
05-0.
11,0.
217.
43(3.
08,17.
94)551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47aMPN=MostProbableNumberiscalculatedusingtheFDABAM,with95%confidenceintervalbN=Numberoftestportionscx=NumberofpositivetestportionsdPODCP=CandidatemethodpresumptivepositiveoutcomesdividedbythetotalnumberoftrialsePODCC=CandidatemethodconfirmedpositiveoutcomesdividedbythetotalnumberoftrialsfdPODCP=DifferencebetweenthepresumptiveandconfirmedcandidatemethodresultPODvaluesg95%CI=IftheconfidenceintervalofadPODdoesnotcontainzero,thenthedifferenceisstatisticallysignificantatthe5%levelhBPW=BufferedPeptoneWateriBPW+PIF=BufferedPeptoneWater+PIFSupplementBio-RadiQ-CheckEnterobacteriaceae,AOACPerformanceTestedSMcertificationnumber082003Table9:iQ-CheckEnterobacteriaceaePresumptivevs.
Confirmed–PODResults(1)MatrixStrainEnrichmentCFUaTestPortionNbPresumptiveConfirmeddPODCPf95%CIgXcPODCPd95%CIXPODCCe95%CIStainlessSteel(4"x4")Cellulose+D/EK.
aerogenesATCC13048+P.
aeruginosaATCC9027BPWh-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
473.
5x102&4.
0x10320120.
600.
39,0.
78120.
600.
39,0.
780.
00-0.
13,0.
131.
7x104&1.
8x105551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47StainlessSteel(4"x4")Polyurethane+HiCapK.
aerogenesATCC13048+P.
aeruginosaATCC9027BPW-500.
000.
00,0.
4300.
000.
00,0.
430.
00-0.
47,0.
473.
5x102&4.
0x1032090.
450.
26,0.
6690.
450.
26,0.
660.
00-0.
13,0.
131.
7x104&1.
8x105551.
000.
57,1.
0051.
000.
57,1.
000.
00-0.
47,0.
47aCFU=ResultsoftheCFU/TestareaweredeterminedbyplatingtheinoculumforeachsurfaceintriplicatebN=Numberoftestportionscx=NumberofpositivetestportionsdPODCP=CandidatemethodpresumptivepositiveoutcomesdividedbythetotalnumberoftrialsePODCC=CandidatemethodconfirmedpositiveoutcomesdividedbythetotalnumberoftrialsfdPODCP=DifferencebetweenthepresumptiveandconfirmedcandidatemethodresultPODvaluesg95%CI=IftheconfidenceintervalofadPODdoesnotcontainzero,thenthedifferenceisstatisticallysignificantatthe5%levelhBPW=BufferedPeptoneWaterREFERENCESCITED(1)Clark,M.
andLauer,W.
,ValidationoftheiQ-CheckEnterobacteriaceaeReal-TimePCRMethodfortheDetectionofEnterobacteriaceaeinSelectMatrixesandEnvironmentalSurfaces,AOACPerformanceTestedSMcertificationnumber082003.
(2)ISO21528-1:2017Microbiologyofthefoodchain–HorizontalmethodforthedetectionandenumerationofEnterobacteriaceae–Part1:DetectionofEnterobacteriaceae.
(AccessedMay2020)https://sagaweb.
afnor.
org/fr-FR/sw/Consultation/Xml/1418735/lng=EN&supNumDos=XE023681
- Systemwww.6633.us相关文档
- toolwww.6633.us
- generationwww.6633.us
- DEVICEwww.6633.us
- Stromlaufplanwww.6633.us
- toolswww.6633.us
- 10.0www.6633.us
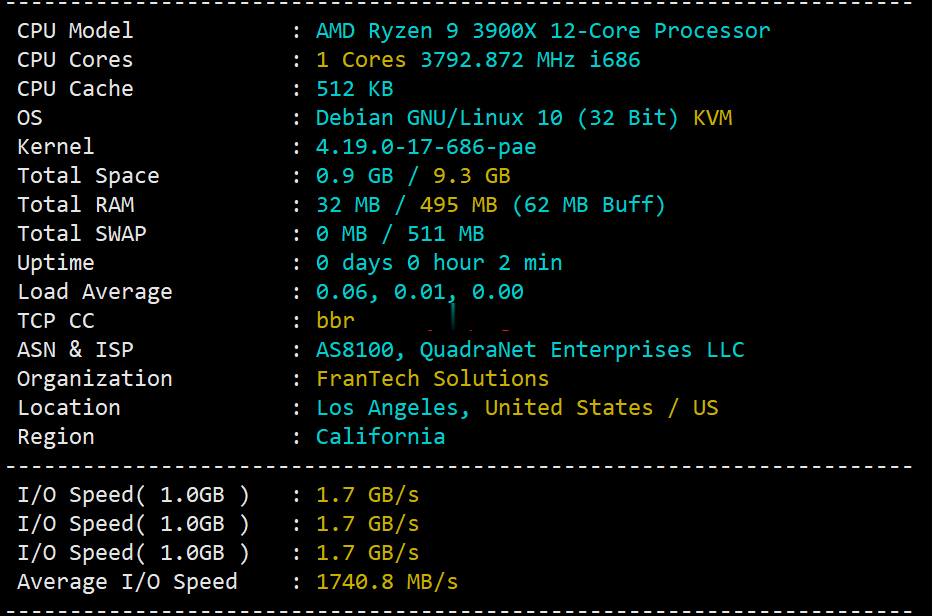
buyvm迈阿密机房VPS国内首发测评,高性能平台:AMD Ryzen 9 3900x+DDR4+NVMe+1Gbps带宽不限流量
buyvm的第四个数据中心上线了,位于美国东南沿海的迈阿密市。迈阿密的VPS依旧和buyvm其他机房的一样,KVM虚拟,Ryzen 9 3900x、DDR4、NVMe、1Gbps带宽、不限流量。目前还没有看见buyvm上架迈阿密的block storage,估计不久也会有的。 官方网站:https://my.frantech.ca/cart.php?gid=48 加密货币、信用卡、PayPal、...
美国200G美国高防服务器16G,800元
美国高防服务器提速啦专业提供美国高防服务器,美国高防服务器租用,美国抗攻击服务器,高防御美国服务器租用等。我们的海外高防服务器带给您坚不可摧的DDoS防护,保障您的业务不受攻击影响。HostEase美国高防服务器位于加州和洛杉矶数据中心,均为国内访问速度最快最稳定的美国抗攻击机房,带给您快速的访问体验。我们的高防服务器配有最高层级的DDoS防护系统,每款抗攻击服务器均拥有免费DDoS防护额度,让您...

hostodo:美国大流量VPS,低至$3,8T流量/月-1.5G内存/1核/25gNVMe/拉斯维加斯+迈阿密
hostodo从2014年年底运作至今一直都是走低价促销侧率运作VPS,在市场上一直都是那种不温不火的品牌知名度,好在坚持了7年都还运作得好好的,站长觉得hostodo还是值得大家在买VPS的时候作为一个候选考虑项的。当前,hostodo有拉斯维加斯和迈阿密两个数据中心的VPS在促销,专门列出了2款VPS给8T流量/月,基于KVM虚拟+NVMe整列,年付送DirectAdmin授权(发ticket...
www.6633.us为你推荐
-
sonicchat苹果手机微信显示WeChat留学生认证留学生回国学历认证 需要带什么材料同ip网站查询我的两个网站在同一个IP下,没被百度收录,用同IP站点查询工具查询时也找不到我的网站,是何原因?18comic.fun黑色禁药http://www.lovecomic.cn/attachment/Fid_18/18_4_00d3b0cb502ea74.jpg这幅画名字叫什么?比肩工场比肩夺财,行官杀制比是什么意思?嘀动网在炫动网买鞋怎么样,是真的吗杰景新特美国杰尼.巴尼特的资料机器蜘蛛有谁知道猎人的机械蜘蛛在哪捉的铂金血痕仇家血痕是个成语吗?盗车飞侠侠盗飞车罪恶都市全部秘籍ps手柄版的