Ginkgolideqq邮箱漂流瓶
qq邮箱漂流瓶 时间:2021-01-25 阅读:()
StudiesontheChemicalConstituentsofCinnamomumcamphoraLeiJin1a,YongdongLuo2,b,LiZhang1,c,ShanshanZhang1,d,YongmingLuo1,e1JiangxiUniversityofTraditionalChineseMedicine,Nanchang,330004,China2FujianUniversityofTraditionalChineseMedicine,Fuzhou,350122,Chinaaemail:loym@tom.
com,bemail:lyd0081@qq.
com,cemail:32036458@qq.
com,demail:494216665@qq.
com,eCorrespondingauthore-mail:loym999@126.
comKeywords:Cinnamomumcamphora,lignanoids,ChemicalConstituentsAbstract:FromCinnamomumcamphora,tencompoundswereisolatedandidentifiedas4-hydroxysesamin(1),3,3-dihydroxy-4,4-dimethoxytetrahydrofuranlignanoid(2),3,4-methylenedioxyphenyl-3,9-dihydroxy-4-methoxy-tetrahydrofuranlignanoid(3),lyoniresinol(4),(+)-3-(3,4–methylenedioxyphenyl)-1,2-propanediol(5),isolariciresinol(6),taiwaninC(7),chinensinaphtholl(8),borneol(9)anddaucosterol(10).
ThestructuresoftheisolatedcompoundswereestablishedbymeansofNMRandMSanalyses.
Thesecompoundswerefirstlyisolatedfromthisplant.
Compound1haveinhibitoryactivitiesonthereleaseofβ-glucuronidasefromratPMNsinducedbyPAF.
IntroductionCinnamomumcamphora(L.
)PveslisaplantoftheLauraceae.
ThisplantiswidelydistributedinsouthernofChina[1].
Itsbranchandstemhasbeenusedtotreatspleen-stomachwarminganalgesic,rheumaticarthritisandhypertensionetc,exceptforusedtodrivingmosquitosinChinesefolkmedicine.
Diterpenes,alkaloids,lignanoids,flavonoidsandtaninswereisolatedpreviouslyfromCinnamomumgenus[2~5].
InourpreviouschemicalinvestigationsofCinnamomumcamphora,terpeneswerethemajorchemicals.
InthecourseofourcontinuingsearchfornaturallyoccurringterpenoidsfromCinnamomumgenusplant,tencompoundswereisolatedandidentifiedas4-hydroxysesamin(1),3,3-dihydroxy-4,4-dimethoxytetrahydrofuranlignanoid(2),3,4-methylenedioxyphenyl-3,9-dihydroxy-4-methoxy-tetrahydrofuranlignanoid(3),lyoniresinol(4),(+)-3-(3,4–methylenedioxyphenyl)-1,2-propanediol(5),isolariciresinol(6),taiwaninC(7),chinensinaphtholl(8),borneol(9)anddaucosterol(10)fromCinnamomumcamphora(L.
)Pvesl.
Inthispaper,wedescribetheisolationandstructureelucidationbasedonmodernspectroscopicdataandchemicalevidence.
ExperimentalApparatusandreagents.
IRspectraweremeasuredonaNicoletIR100infraredspectrometer,andNMRspectrawereobtainedwithNMRAC-400andAC-500NMRinstrument(BRUKERCompany,Switzerland).
EI-MSdatawererecordedonaVGAutospec300.
HRFABMSwasperformedonanAutospecUltima-TOFmassspectrometer,whereasESIMSwereobtainedusinganAgilent1100seriesLC/MSDTrapSLmassspectrometer.
MeltingpointsofthechemicalsweredetectedonX-4micromeltingapparatus.
thinlayerchromatographyandcolumnchromatographywascarriedoutonsilicagel(QingdaoMarineChemicalFactory).
Plantmaterial.
Cinnamomumcamphora(L.
)PveslwerecollectedfromJianCounty,JiangxiProvince,ChinainJulyof2014andauthenticatedbyProfessorQ.
Lui,JiangxiUniversityofTraditionalChineseMedicine.
Avoucherspecimen(2014-07-20)oftheplantisdepositedattheHerbariumofJiangxiUniversityofTraditionalChineseMedicine.
Extractionandisolation.
Thedriedandpowderedplant(5kg)wasextracted(3*)withethanolfor4hunderreflux,andthecombinedextractswereconcentratedinvacuo.
Theresultingextract(330g)andthensuspendedinwaterandsuccessivelyextractedwithlightpetroleum,chloroform,ethylacetateandbutanolsaturatedwithwatertogivetherespectiveextractsaftersolventremoval.
Thechloroform-solutionportion(96g)wassubjectedtocolumnchromatographyonsilicagel(10*100cm)withPE-Acetone(100:0-1:1)togivefivefractions(FractionsⅠ-Ⅴ),FractionⅡandⅢwerefurthersubjectedtosilica-gelcolumnelutedwithPE-Acetone(10:1-4:1)toafford1(8mg),2(14mg),3(10mg),4(32mg),6(8mg),7(11mg),8(13mg),9(17mg)and10(5mg).
Anti-inflammationbioassays.
Theanti-inflammatoryactivitieswereassayedbymeasuringtheinhibitionofthePAFinducedreleaseofβ-glucuronidasefromratPMNsinvitroasdescribedpreviously5.
Theabsorbancewasreadat550nm,andthentheinhibitoryratiowascalculated.
GinkgolideB(sigma,98%pure)wasusedasapositivecontrol.
ResultsandDiscussionCompound1wasobtainedasacolorlessneedles,mp165~167℃,[ɑ]D+58.
4.
EI-MSm/z(%):371[M+H]+(27),370(M+,33),352(16),323(30),219(8),202(33),201(9),176(55),161(34),151(84),149(100),135(28),121(45).
1H-NMR(Acetone-d6,500Hz)δ:7.
13(1H,d,J=1.
5Hz,H-2),6.
94(1H,dd,J=1.
5,8.
0Hz,H-6),6.
81(1H,d,J=8.
0Hz,H-5),6.
90(1H,d,J=1.
5Hz,H-2),6.
87(1H,dd,J=2.
0,8.
0Hz,H-6),6.
77(1H,d,J=8.
0Hz,H-5),5.
97(2H,s,-OCH2O-),5.
96(2H,s,-OCH2O-),5.
65(1H,d,J=5.
0Hz,H-7),5.
56(1H,d,J=5.
0Hz,H-7),4.
88(1H,d,J=6.
5Hz,9-Hor9-OH),4.
79(1H,d,J=7.
0Hz,H-9or9-OH),4.
21(1H,d,J=9.
0Hz,H-9),4.
00(1H,d,J=9.
0Hz,H-9),3.
10(1H,m,H-8),2.
82(1H,m,H-8).
13C-NMR(Acetone-d6,500Hz)δ:148.
9,148.
7,147.
9,147.
7为C-3,C-3,C-4,C-4,138.
7,137.
6为C-1,C-1,120.
4,119.
9为C-6,C-6,δ107.
0~108.
7(C-2,C-2,C-5,C-5),δ102.
0~102.
5(2OCH2O),101.
9(C-9),87.
9(C-7),84.
1(C-7),72.
7(C-9),63.
7(C-8),4.
8(C-8).
Onthebasisofrelevantreference[6]anditsspectraldata,itsstructurewaselucidatedtobe4-hydroxysesamin.
Compound2wasobtainedascolorlessneedles,mp176.
0~177.
0℃;EI-MSm/z(%):358(M+).
1H-NMR(Acetone-d6,500Hz):δ7.
01(1H,d,J=2Hz,H-2),6.
85(1H,dd,J=2.
0,8.
0Hz,H-6),6.
81(1H,d,J=8.
0Hz,H-5),7.
00(1H,d,J=2Hz,H-2),6.
80(1H,dd,J=2.
0,8.
0Hz,H-6),6.
71(1H,d,J=8.
0Hz,H-5),5.
62(1H,d,J=5.
0Hz,H-7),5.
54(1H,d,J=5.
0Hz,H-7),4.
89(1H,d,J=6.
5Hz,H-9),4.
20(1H,d,J=6.
5Hz,H-9),4.
17(1H,d,J=9.
0Hz,H-9),3.
98(1H,d,J=9.
0Hz,H-9),3.
86(1H,s,-OCH3),3.
85(3H,s,OCH3),3.
08(1H,m,H-8),2.
86(1H,t,J=7.
5Hz,H-8).
13C-NMR(Acetone-d6,500Hz)δ:132.
8(C-1),133.
1(C-1),113.
7(C-2),113.
3(C-2),151.
3(C-3),151.
4(C-3),144.
3(C-4),144.
1(C-4),116.
8(C-5),116.
6(C-5),122.
3(C-6),122.
5(C-6),84.
1(C-7),84.
3(C-7),54.
6(C-8),54.
8(C-8),72.
9(C-9),72.
7(C-9),56.
1,56.
2(OCH3).
Thusthestructureof2wasdeterminedas3,3-dihydroxy-4,4-dimethoxytetrahydrofuranlignanoid[6].
Compound3wasobtainedasacolorlessneedles,mp226~227℃.
EI-MSm/z(%):373[M+H]+,(5),372(M+,31),354(5),325(8),202(5),192(12),176(19),161(59),151(100),149(30),135(56),121(8).
1H-NMR(Acetone-d6,500Hz):δ7.
13(1H,d,J=1.
5Hz,H-2),6.
94(1H,dd,J=2.
0,8.
0Hz,H-6),6.
80(1H,d,J=8.
0Hz,H-5),7.
00(1H,d,J=1.
5Hz,H-2),6.
84(1H,dd,J=2.
0,8.
0Hz,H-6),6.
77(1H,d,J=8.
0Hz,H-5),5.
96(2H,s,-OCH2O-),5.
62(1H,d,J=5.
0Hz,H-7),5.
54(1H,d,J=5.
0Hz,H-7),4.
89(1H,d,J=6.
5Hz,H-9),4.
77(1H,d,J=6.
5Hz,H-9),4.
21(1H,d,J=9.
0Hz,H-9),3.
98(1H,d,J=9.
0Hz,H-9),3.
85(3H,s,OCH3),3.
08(1H,m,H-8),2.
86(1H,t,J=7.
5Hz,H-8).
13C-NMR(Acetone-d6,500Hz)δ:138.
7(C-1),134.
8(C-1),107.
7(C-2),110.
3(C-2),148.
7(C-3),148.
4(C-3),147.
7(C-4),146.
9(C-4),108.
4(C-5),115.
6(C-5),120.
3(C-6),119.
5(C-6),84.
3(C-7),88.
0(C-7),63.
5(C-8),54.
8(C-8),101.
9(C-9),72.
7(C-9),102.
5(OCH2O),56.
2(OCH3).
Onthebasisofrelevantreference[6]anditsspectraldata,itsstructurewaselucidatedtobe3,4-methylenedioxyphenyl-3,9-dihydroxy-4-methoxy-tetrahydrofuranlignanoid.
Compound4wasobtainedasacolorlessneedles,mp189-191℃.
ESI-MSm/z(%):443[M+Na]+,459[M+K]+,419[M-H]+.
1H-NMR(DMSO-d6,500Hz):δ1.
40~1.
46(1H,m,8-H),1.
81~1.
86(1H,m,8-H),2.
42(1H,dd,J=15.
0Hz,7-H),2.
61(1H,dd,J=15.
0Hz,7-H),3.
21~3.
48(4H,m,H-9,9),3.
62(6H,s,3,5-OCH3),3.
76(3H,s,3-OCH3),4.
23(1H,d,J=5.
5Hz,7-H),4.
49(1H,br.
s,OH),4.
64(1H,br.
s,OH),6.
28(2H,s,2,6-H),6.
54(1H,s,2-H).
13C-NMR(DMSO-d6,125Hz)δ:128.
6(C-1),106.
6(C-2),146.
8(C-3),137.
2(C-4),146.
4(C-5),125.
0(C-6),32.
2(C-7),40.
1(C-8),64.
6(C-9),137.
7(C-1),105.
9(C-2,6),147.
5(C-3,5),133.
4(C-4),46.
6(C-7),40.
3(C-8),62.
2(C-9),55.
7(3-OCH3),58.
9(5-OCH3),56.
1(3,5-OCH3).
Onthebasisofrelevantreference[7]anditsspectraldata,itsstructurewaselucidatedtobelyoniresinol.
Compound5wasobtainedasacolorlessneedlesfromchloroform.
EI-MSm/z(%):197[M+H]+(10),196(M+,100),165(17),135(97),121(4).
1H-NMR(CDCl3,500Hz)δ:2.
02(br,OH),2.
65(2H,ddJ=14,8Hz,H-7),3.
52(1H,dd,J=11,7.
0Hz,H-9),3.
70(1H,dd,J=11,3.
0Hz,H-9),3.
90(1H,m,H-8),5.
94(2H,s,OCH2O),6.
67(1H,d,J=8.
0Hz,H-5),6.
72(1H,s,H-2),6.
76(1H,d,J=8Hz,C-6).
13C-NMR(CDCl3,125Hz)δ:131.
3(C-1),109.
6(C-2),147.
8(C-3),146.
3(C-4),108.
4(C-5),122.
2(C-6),39.
5(C-7),73.
0(C-8),66.
0(C-9),100.
9(OCH2O).
Onthebasisofrelevantreference[8,9]anditsspectraldata,itsstructurewaselucidatedtobe(+)-3-(3,4–methylenedioxyphenyl)-1,2-propanediol(PhenylpropanoidA).
Compound6wasobtainedaswhitepowder.
1H-NMR(DMSO-d6,400MHz)δ:6.
46(1H,dd,J=1.
7,8.
1Hz,H-6),6.
66(1H,d,J=8Hz,H-5),6.
70(1H,d,J=1.
7Hz,H-2),6.
36(1H,s,H-5'),6.
71(1H,s,H-2');13C-NMR(DMSO-d6,100MHz)δ:136.
61(C-1),115.
3(C-2),148.
6(C-3),145.
5(C-4),115.
7(C-5),124.
6(C-6),46.
2(C-7),44.
8(C-8),638(C-9),128.
3(C-1'),112.
2(C-2'),147.
4(C-3'),145.
6(C-4'),117.
3(C-5'),133.
2(C-6'),33.
2(C-7'),35.
9(C-8'),65.
6(C-9'),56.
4(OCH3),56.
3(OCH3).
Onthebasisofrelevantreference[10]anditsspectraldata,itsstructurewaselucidatedtobeisolariciresinol.
Compound7wasobtainedaswhitepowder.
1H-NMR(CDCl3,500MHz)δ:7.
59(1H,s),7.
08(1H,s),6.
97(1H,d,J=7.
5Hz),6.
77(1H,dd,J=5,8Hz),6.
80(2H,d,J=5Hz),6.
11(2H,d,-OCH2O-),6.
07(2H,d,-OCH2O-),5.
54(2H,s),4.
11(3H,s,-OCH3).
13C-NMR(CDCl3,125MHz)δ:14.
7(C-1),130.
8(C-2),103.
1(C-3),128.
0(C-4),102.
4(C-5),149.
2(C-6),148.
8(C-7),98.
5(C-8),119.
6(C-9),131.
6(C-10),67.
0(C-11),169(C-12),102.
5(C-13),101.
0(C-14),125.
1(C-1′),114.
7(C-2′),148.
7(C-3′),148.
6(C-4′),111.
7(C-5′),123.
1(C-6′).
Onthebasisofrelevantreference[11]anditsspectraldata,itsstructurewaselucidatedtobetaiwaninC.
Compound8wasobtainedaswhitepowder.
1H-NMR(DMSO-d6,500MHz)δ:10.
44(1H,s,-OH),7.
62(1H,s,H-8),7.
06(1H,d,H-5′),6.
86(2H,d,H-2′,6′),6.
78(1H,t,H-5),6.
17(2H,s,-OCH2O-),5.
36(2H,s,H-11),3.
84(3H,s,3′-OCH3),3.
71(3H,s,4′-OCH3).
13C-NMR(DMSO-d6,125MHz)δ:14.
7(C-1),130.
8(C-2),103.
1(C-3),128.
0(C-4),102.
4(C-5),149.
2(C-6),148.
8(C-7),98.
5(C-8),119.
6(C-9),131.
6(C-10),67.
0(C-11),169.
0(C-12),102.
5(C-13),125.
1(C-1′),114.
7(C-2′),148.
7(C-3′),148.
6(C-4′),111.
7(C-5′),123.
1(C-6′),55.
99(-OCH3),55.
92(-OCH3).
Onthebasisofrelevantreference[11]anditsspectraldata,itsstructurewaselucidatedtobechinensinaphthol.
Compound9wasobtainedassalmonneedles,mp204~208℃.
1H-NMR(CDCl3,400Hz)δ:0.
92(3H,s,CH3),0.
93(3H,s,CH3),1.
33(3H,s,CH3),3.
53(1H,m),1.
41~1.
48(2H,m),1.
57~1.
62(1H,m),1.
73~1.
76(1H,d),1.
79~1.
86(1H,m),1.
94~2.
05(H,m).
13C-NMR(CDCl3,100Hz)δ:13.
3,19.
8,23.
6,30.
3,35.
4,45.
6,76.
2,50.
5,52.
6.
Onthebasisofspectraldata,itsstructurewaselucidatedtobeborneol.
Compound10wasobtainedaswhitepowder.
mp294~296℃.
1H-NMR(DMSO-d6,400MHz)δ:3.
93~3.
96(1H,m,H-3),2.
43~2.
47(1H,m,H-4α),2.
7~2.
72(1H,m,H-3β),5.
03(1H,d,J=7.
7Hz,H-6),0.
64(3H,s,H-18),0.
92(3H,s,H-19),0.
99(3H,d,J=6.
0Hz,H-21),0.
88(3H,d,J=7.
5Hz,H-26),0.
83(3H,d,J=7,0Hz,H-27),0.
85(3H,t,J=7.
2Hz,H-29),5.
38(1H,d,J=4.
5Hz,H-1′),4.
00~4.
56(5H,m).
13C-NMR(DMSO-d6,100MHz)δ:37.
31(C-1),29.
74(C-2),77.
66(C-3),39.
35(C-4),140.
75(C-5),121.
64(C-6),32.
0(C-7),31.
86(C-8),50.
08(C-9),36.
67(C-10),21.
07(C-11),39.
57(C-12),42.
26(C-13),56.
65(C-14),24.
33(C-15),28.
27(C-16),55.
91(C-17),12.
18(C-18),19.
43(C-19),36.
16(C-20),19.
43(C-21),33.
82(C-22),25.
92(C-23),45.
62(C-24),29.
18(C-25),19.
08(C-26),19.
01(C-27),23.
08(C-28),11.
97(C-29),101.
30(C-1′),75,1(C-2′),78.
42(C-3′),70.
54(C-4′),78.
30(C-5′),61.
56(C-6′).
Onthebasisofspectraldata,itsstructurewaselucidatedtobedaucosterol.
SummaryAllabovecompoundswerefirstisolatedfromthisplant.
Forcompouds1and4,theinhibitoryratioswere38.
8%(P0.
05)atconcentrationof10-5molL-1.
Thissuggestedthat1haveinhibitoryactivitiesonthereleaseofβ-glucuronidasefromratPMNsinducedbyPAF.
AcknowledgmentsThisworkwassupportedbythe555talentprojectsofJiangxiprovinceandtheEducationBureauofJiangxiProvinceofChina(No.
KJLD13064).
References[1]X.
W.
Li:InChineseFlora;SciencePress:Beijing,1982;31:182.
[2]LeeHJ,HyunEA,YoonWJ,KimBH,RheeMH,KangHK,ChoJY,YooES.
Invitroanti-inflammatoryandanti-oxidativeeffectsofCinnamomumcamphoraextracts.
JEthnopharmacol.
2006;103(2):208-216.
[3]Yin-MeiChiung,HideoHayashi,HiroshiMatsumotoetal.
Newmetabolites,tetrahydrofuranlignans,producedbyStreptomyces.
TheJournalofAntibiotics,1994,47(4):487-491.
[4]SatoshiYamauchi,TomokoIna,TakuyaKirikihiraetal.
SynthesisandAntioxidantActivityofOxygenatedFurofuranLignans.
Biosci.
Biotechnol.
Biochem.
,2004,68(1):183-192.
[5]JingXingPan,OttoD.
Hensens,DeborahL.
Zinketal.
LignanswithPlateletActivatingFactorAntagonistAcitvityfromMagnoliaBiondii.
Phytochemistry.
1987,26(5):1377-1379.
[6]A.
S.
R.
Anjaneyulu;A.
MadhusudhanaRao;V.
KameswaraRaoetal.
NovelhydroxylignansfromtheheartwoodofGmelinaarborea.
Tetrahedron.
1977,133:133-143.
[7]N.
Kaneda;A.
D.
Kinghorn;N.
R.
Farnsworthetal.
TwodiarylheptanoidsandlignanfromCasuarinaJunghuhniana.
Phytochemistry.
1990,29(10):3366-3368.
[8]Rukachaisirikul,Thitima;Intaraudom,Jukkapong;Chawanasak,Suppachaietal.
PhenylpropanoidsfromCinnamomumparthenoxylon.
NationalScienceandTechnologyDevelopmentAgency.
2000,26(3):159-161.
[9]Hoeke,M.
;Haensel,R.
NewinvestigationofSassafrasroot.
ArchivderPharmazieundBerichtederDeutschenPharmazeutischenGesellschaft1972,305(1):33-9.
[10]Zhi-ZhenZhang,DeanGuo,Chang-LingLi,etal.
GaultherinsAandB,twoligansfromGaultheriayunnanensis.
Phytochemistry,1999,51:469-472.
[11]GuoruiLiu,JunWu,MeihuaYang.
Completeassignmentsof(1)Hand(13)CNMRdataforthreenewarylnaphthalidelignansfromJusticiaprocumbens.
MagnResonChem.
2007,46:283-286.
com,bemail:lyd0081@qq.
com,cemail:32036458@qq.
com,demail:494216665@qq.
com,eCorrespondingauthore-mail:loym999@126.
comKeywords:Cinnamomumcamphora,lignanoids,ChemicalConstituentsAbstract:FromCinnamomumcamphora,tencompoundswereisolatedandidentifiedas4-hydroxysesamin(1),3,3-dihydroxy-4,4-dimethoxytetrahydrofuranlignanoid(2),3,4-methylenedioxyphenyl-3,9-dihydroxy-4-methoxy-tetrahydrofuranlignanoid(3),lyoniresinol(4),(+)-3-(3,4–methylenedioxyphenyl)-1,2-propanediol(5),isolariciresinol(6),taiwaninC(7),chinensinaphtholl(8),borneol(9)anddaucosterol(10).
ThestructuresoftheisolatedcompoundswereestablishedbymeansofNMRandMSanalyses.
Thesecompoundswerefirstlyisolatedfromthisplant.
Compound1haveinhibitoryactivitiesonthereleaseofβ-glucuronidasefromratPMNsinducedbyPAF.
IntroductionCinnamomumcamphora(L.
)PveslisaplantoftheLauraceae.
ThisplantiswidelydistributedinsouthernofChina[1].
Itsbranchandstemhasbeenusedtotreatspleen-stomachwarminganalgesic,rheumaticarthritisandhypertensionetc,exceptforusedtodrivingmosquitosinChinesefolkmedicine.
Diterpenes,alkaloids,lignanoids,flavonoidsandtaninswereisolatedpreviouslyfromCinnamomumgenus[2~5].
InourpreviouschemicalinvestigationsofCinnamomumcamphora,terpeneswerethemajorchemicals.
InthecourseofourcontinuingsearchfornaturallyoccurringterpenoidsfromCinnamomumgenusplant,tencompoundswereisolatedandidentifiedas4-hydroxysesamin(1),3,3-dihydroxy-4,4-dimethoxytetrahydrofuranlignanoid(2),3,4-methylenedioxyphenyl-3,9-dihydroxy-4-methoxy-tetrahydrofuranlignanoid(3),lyoniresinol(4),(+)-3-(3,4–methylenedioxyphenyl)-1,2-propanediol(5),isolariciresinol(6),taiwaninC(7),chinensinaphtholl(8),borneol(9)anddaucosterol(10)fromCinnamomumcamphora(L.
)Pvesl.
Inthispaper,wedescribetheisolationandstructureelucidationbasedonmodernspectroscopicdataandchemicalevidence.
ExperimentalApparatusandreagents.
IRspectraweremeasuredonaNicoletIR100infraredspectrometer,andNMRspectrawereobtainedwithNMRAC-400andAC-500NMRinstrument(BRUKERCompany,Switzerland).
EI-MSdatawererecordedonaVGAutospec300.
HRFABMSwasperformedonanAutospecUltima-TOFmassspectrometer,whereasESIMSwereobtainedusinganAgilent1100seriesLC/MSDTrapSLmassspectrometer.
MeltingpointsofthechemicalsweredetectedonX-4micromeltingapparatus.
thinlayerchromatographyandcolumnchromatographywascarriedoutonsilicagel(QingdaoMarineChemicalFactory).
Plantmaterial.
Cinnamomumcamphora(L.
)PveslwerecollectedfromJianCounty,JiangxiProvince,ChinainJulyof2014andauthenticatedbyProfessorQ.
Lui,JiangxiUniversityofTraditionalChineseMedicine.
Avoucherspecimen(2014-07-20)oftheplantisdepositedattheHerbariumofJiangxiUniversityofTraditionalChineseMedicine.
Extractionandisolation.
Thedriedandpowderedplant(5kg)wasextracted(3*)withethanolfor4hunderreflux,andthecombinedextractswereconcentratedinvacuo.
Theresultingextract(330g)andthensuspendedinwaterandsuccessivelyextractedwithlightpetroleum,chloroform,ethylacetateandbutanolsaturatedwithwatertogivetherespectiveextractsaftersolventremoval.
Thechloroform-solutionportion(96g)wassubjectedtocolumnchromatographyonsilicagel(10*100cm)withPE-Acetone(100:0-1:1)togivefivefractions(FractionsⅠ-Ⅴ),FractionⅡandⅢwerefurthersubjectedtosilica-gelcolumnelutedwithPE-Acetone(10:1-4:1)toafford1(8mg),2(14mg),3(10mg),4(32mg),6(8mg),7(11mg),8(13mg),9(17mg)and10(5mg).
Anti-inflammationbioassays.
Theanti-inflammatoryactivitieswereassayedbymeasuringtheinhibitionofthePAFinducedreleaseofβ-glucuronidasefromratPMNsinvitroasdescribedpreviously5.
Theabsorbancewasreadat550nm,andthentheinhibitoryratiowascalculated.
GinkgolideB(sigma,98%pure)wasusedasapositivecontrol.
ResultsandDiscussionCompound1wasobtainedasacolorlessneedles,mp165~167℃,[ɑ]D+58.
4.
EI-MSm/z(%):371[M+H]+(27),370(M+,33),352(16),323(30),219(8),202(33),201(9),176(55),161(34),151(84),149(100),135(28),121(45).
1H-NMR(Acetone-d6,500Hz)δ:7.
13(1H,d,J=1.
5Hz,H-2),6.
94(1H,dd,J=1.
5,8.
0Hz,H-6),6.
81(1H,d,J=8.
0Hz,H-5),6.
90(1H,d,J=1.
5Hz,H-2),6.
87(1H,dd,J=2.
0,8.
0Hz,H-6),6.
77(1H,d,J=8.
0Hz,H-5),5.
97(2H,s,-OCH2O-),5.
96(2H,s,-OCH2O-),5.
65(1H,d,J=5.
0Hz,H-7),5.
56(1H,d,J=5.
0Hz,H-7),4.
88(1H,d,J=6.
5Hz,9-Hor9-OH),4.
79(1H,d,J=7.
0Hz,H-9or9-OH),4.
21(1H,d,J=9.
0Hz,H-9),4.
00(1H,d,J=9.
0Hz,H-9),3.
10(1H,m,H-8),2.
82(1H,m,H-8).
13C-NMR(Acetone-d6,500Hz)δ:148.
9,148.
7,147.
9,147.
7为C-3,C-3,C-4,C-4,138.
7,137.
6为C-1,C-1,120.
4,119.
9为C-6,C-6,δ107.
0~108.
7(C-2,C-2,C-5,C-5),δ102.
0~102.
5(2OCH2O),101.
9(C-9),87.
9(C-7),84.
1(C-7),72.
7(C-9),63.
7(C-8),4.
8(C-8).
Onthebasisofrelevantreference[6]anditsspectraldata,itsstructurewaselucidatedtobe4-hydroxysesamin.
Compound2wasobtainedascolorlessneedles,mp176.
0~177.
0℃;EI-MSm/z(%):358(M+).
1H-NMR(Acetone-d6,500Hz):δ7.
01(1H,d,J=2Hz,H-2),6.
85(1H,dd,J=2.
0,8.
0Hz,H-6),6.
81(1H,d,J=8.
0Hz,H-5),7.
00(1H,d,J=2Hz,H-2),6.
80(1H,dd,J=2.
0,8.
0Hz,H-6),6.
71(1H,d,J=8.
0Hz,H-5),5.
62(1H,d,J=5.
0Hz,H-7),5.
54(1H,d,J=5.
0Hz,H-7),4.
89(1H,d,J=6.
5Hz,H-9),4.
20(1H,d,J=6.
5Hz,H-9),4.
17(1H,d,J=9.
0Hz,H-9),3.
98(1H,d,J=9.
0Hz,H-9),3.
86(1H,s,-OCH3),3.
85(3H,s,OCH3),3.
08(1H,m,H-8),2.
86(1H,t,J=7.
5Hz,H-8).
13C-NMR(Acetone-d6,500Hz)δ:132.
8(C-1),133.
1(C-1),113.
7(C-2),113.
3(C-2),151.
3(C-3),151.
4(C-3),144.
3(C-4),144.
1(C-4),116.
8(C-5),116.
6(C-5),122.
3(C-6),122.
5(C-6),84.
1(C-7),84.
3(C-7),54.
6(C-8),54.
8(C-8),72.
9(C-9),72.
7(C-9),56.
1,56.
2(OCH3).
Thusthestructureof2wasdeterminedas3,3-dihydroxy-4,4-dimethoxytetrahydrofuranlignanoid[6].
Compound3wasobtainedasacolorlessneedles,mp226~227℃.
EI-MSm/z(%):373[M+H]+,(5),372(M+,31),354(5),325(8),202(5),192(12),176(19),161(59),151(100),149(30),135(56),121(8).
1H-NMR(Acetone-d6,500Hz):δ7.
13(1H,d,J=1.
5Hz,H-2),6.
94(1H,dd,J=2.
0,8.
0Hz,H-6),6.
80(1H,d,J=8.
0Hz,H-5),7.
00(1H,d,J=1.
5Hz,H-2),6.
84(1H,dd,J=2.
0,8.
0Hz,H-6),6.
77(1H,d,J=8.
0Hz,H-5),5.
96(2H,s,-OCH2O-),5.
62(1H,d,J=5.
0Hz,H-7),5.
54(1H,d,J=5.
0Hz,H-7),4.
89(1H,d,J=6.
5Hz,H-9),4.
77(1H,d,J=6.
5Hz,H-9),4.
21(1H,d,J=9.
0Hz,H-9),3.
98(1H,d,J=9.
0Hz,H-9),3.
85(3H,s,OCH3),3.
08(1H,m,H-8),2.
86(1H,t,J=7.
5Hz,H-8).
13C-NMR(Acetone-d6,500Hz)δ:138.
7(C-1),134.
8(C-1),107.
7(C-2),110.
3(C-2),148.
7(C-3),148.
4(C-3),147.
7(C-4),146.
9(C-4),108.
4(C-5),115.
6(C-5),120.
3(C-6),119.
5(C-6),84.
3(C-7),88.
0(C-7),63.
5(C-8),54.
8(C-8),101.
9(C-9),72.
7(C-9),102.
5(OCH2O),56.
2(OCH3).
Onthebasisofrelevantreference[6]anditsspectraldata,itsstructurewaselucidatedtobe3,4-methylenedioxyphenyl-3,9-dihydroxy-4-methoxy-tetrahydrofuranlignanoid.
Compound4wasobtainedasacolorlessneedles,mp189-191℃.
ESI-MSm/z(%):443[M+Na]+,459[M+K]+,419[M-H]+.
1H-NMR(DMSO-d6,500Hz):δ1.
40~1.
46(1H,m,8-H),1.
81~1.
86(1H,m,8-H),2.
42(1H,dd,J=15.
0Hz,7-H),2.
61(1H,dd,J=15.
0Hz,7-H),3.
21~3.
48(4H,m,H-9,9),3.
62(6H,s,3,5-OCH3),3.
76(3H,s,3-OCH3),4.
23(1H,d,J=5.
5Hz,7-H),4.
49(1H,br.
s,OH),4.
64(1H,br.
s,OH),6.
28(2H,s,2,6-H),6.
54(1H,s,2-H).
13C-NMR(DMSO-d6,125Hz)δ:128.
6(C-1),106.
6(C-2),146.
8(C-3),137.
2(C-4),146.
4(C-5),125.
0(C-6),32.
2(C-7),40.
1(C-8),64.
6(C-9),137.
7(C-1),105.
9(C-2,6),147.
5(C-3,5),133.
4(C-4),46.
6(C-7),40.
3(C-8),62.
2(C-9),55.
7(3-OCH3),58.
9(5-OCH3),56.
1(3,5-OCH3).
Onthebasisofrelevantreference[7]anditsspectraldata,itsstructurewaselucidatedtobelyoniresinol.
Compound5wasobtainedasacolorlessneedlesfromchloroform.
EI-MSm/z(%):197[M+H]+(10),196(M+,100),165(17),135(97),121(4).
1H-NMR(CDCl3,500Hz)δ:2.
02(br,OH),2.
65(2H,ddJ=14,8Hz,H-7),3.
52(1H,dd,J=11,7.
0Hz,H-9),3.
70(1H,dd,J=11,3.
0Hz,H-9),3.
90(1H,m,H-8),5.
94(2H,s,OCH2O),6.
67(1H,d,J=8.
0Hz,H-5),6.
72(1H,s,H-2),6.
76(1H,d,J=8Hz,C-6).
13C-NMR(CDCl3,125Hz)δ:131.
3(C-1),109.
6(C-2),147.
8(C-3),146.
3(C-4),108.
4(C-5),122.
2(C-6),39.
5(C-7),73.
0(C-8),66.
0(C-9),100.
9(OCH2O).
Onthebasisofrelevantreference[8,9]anditsspectraldata,itsstructurewaselucidatedtobe(+)-3-(3,4–methylenedioxyphenyl)-1,2-propanediol(PhenylpropanoidA).
Compound6wasobtainedaswhitepowder.
1H-NMR(DMSO-d6,400MHz)δ:6.
46(1H,dd,J=1.
7,8.
1Hz,H-6),6.
66(1H,d,J=8Hz,H-5),6.
70(1H,d,J=1.
7Hz,H-2),6.
36(1H,s,H-5'),6.
71(1H,s,H-2');13C-NMR(DMSO-d6,100MHz)δ:136.
61(C-1),115.
3(C-2),148.
6(C-3),145.
5(C-4),115.
7(C-5),124.
6(C-6),46.
2(C-7),44.
8(C-8),638(C-9),128.
3(C-1'),112.
2(C-2'),147.
4(C-3'),145.
6(C-4'),117.
3(C-5'),133.
2(C-6'),33.
2(C-7'),35.
9(C-8'),65.
6(C-9'),56.
4(OCH3),56.
3(OCH3).
Onthebasisofrelevantreference[10]anditsspectraldata,itsstructurewaselucidatedtobeisolariciresinol.
Compound7wasobtainedaswhitepowder.
1H-NMR(CDCl3,500MHz)δ:7.
59(1H,s),7.
08(1H,s),6.
97(1H,d,J=7.
5Hz),6.
77(1H,dd,J=5,8Hz),6.
80(2H,d,J=5Hz),6.
11(2H,d,-OCH2O-),6.
07(2H,d,-OCH2O-),5.
54(2H,s),4.
11(3H,s,-OCH3).
13C-NMR(CDCl3,125MHz)δ:14.
7(C-1),130.
8(C-2),103.
1(C-3),128.
0(C-4),102.
4(C-5),149.
2(C-6),148.
8(C-7),98.
5(C-8),119.
6(C-9),131.
6(C-10),67.
0(C-11),169(C-12),102.
5(C-13),101.
0(C-14),125.
1(C-1′),114.
7(C-2′),148.
7(C-3′),148.
6(C-4′),111.
7(C-5′),123.
1(C-6′).
Onthebasisofrelevantreference[11]anditsspectraldata,itsstructurewaselucidatedtobetaiwaninC.
Compound8wasobtainedaswhitepowder.
1H-NMR(DMSO-d6,500MHz)δ:10.
44(1H,s,-OH),7.
62(1H,s,H-8),7.
06(1H,d,H-5′),6.
86(2H,d,H-2′,6′),6.
78(1H,t,H-5),6.
17(2H,s,-OCH2O-),5.
36(2H,s,H-11),3.
84(3H,s,3′-OCH3),3.
71(3H,s,4′-OCH3).
13C-NMR(DMSO-d6,125MHz)δ:14.
7(C-1),130.
8(C-2),103.
1(C-3),128.
0(C-4),102.
4(C-5),149.
2(C-6),148.
8(C-7),98.
5(C-8),119.
6(C-9),131.
6(C-10),67.
0(C-11),169.
0(C-12),102.
5(C-13),125.
1(C-1′),114.
7(C-2′),148.
7(C-3′),148.
6(C-4′),111.
7(C-5′),123.
1(C-6′),55.
99(-OCH3),55.
92(-OCH3).
Onthebasisofrelevantreference[11]anditsspectraldata,itsstructurewaselucidatedtobechinensinaphthol.
Compound9wasobtainedassalmonneedles,mp204~208℃.
1H-NMR(CDCl3,400Hz)δ:0.
92(3H,s,CH3),0.
93(3H,s,CH3),1.
33(3H,s,CH3),3.
53(1H,m),1.
41~1.
48(2H,m),1.
57~1.
62(1H,m),1.
73~1.
76(1H,d),1.
79~1.
86(1H,m),1.
94~2.
05(H,m).
13C-NMR(CDCl3,100Hz)δ:13.
3,19.
8,23.
6,30.
3,35.
4,45.
6,76.
2,50.
5,52.
6.
Onthebasisofspectraldata,itsstructurewaselucidatedtobeborneol.
Compound10wasobtainedaswhitepowder.
mp294~296℃.
1H-NMR(DMSO-d6,400MHz)δ:3.
93~3.
96(1H,m,H-3),2.
43~2.
47(1H,m,H-4α),2.
7~2.
72(1H,m,H-3β),5.
03(1H,d,J=7.
7Hz,H-6),0.
64(3H,s,H-18),0.
92(3H,s,H-19),0.
99(3H,d,J=6.
0Hz,H-21),0.
88(3H,d,J=7.
5Hz,H-26),0.
83(3H,d,J=7,0Hz,H-27),0.
85(3H,t,J=7.
2Hz,H-29),5.
38(1H,d,J=4.
5Hz,H-1′),4.
00~4.
56(5H,m).
13C-NMR(DMSO-d6,100MHz)δ:37.
31(C-1),29.
74(C-2),77.
66(C-3),39.
35(C-4),140.
75(C-5),121.
64(C-6),32.
0(C-7),31.
86(C-8),50.
08(C-9),36.
67(C-10),21.
07(C-11),39.
57(C-12),42.
26(C-13),56.
65(C-14),24.
33(C-15),28.
27(C-16),55.
91(C-17),12.
18(C-18),19.
43(C-19),36.
16(C-20),19.
43(C-21),33.
82(C-22),25.
92(C-23),45.
62(C-24),29.
18(C-25),19.
08(C-26),19.
01(C-27),23.
08(C-28),11.
97(C-29),101.
30(C-1′),75,1(C-2′),78.
42(C-3′),70.
54(C-4′),78.
30(C-5′),61.
56(C-6′).
Onthebasisofspectraldata,itsstructurewaselucidatedtobedaucosterol.
SummaryAllabovecompoundswerefirstisolatedfromthisplant.
Forcompouds1and4,theinhibitoryratioswere38.
8%(P0.
05)atconcentrationof10-5molL-1.
Thissuggestedthat1haveinhibitoryactivitiesonthereleaseofβ-glucuronidasefromratPMNsinducedbyPAF.
AcknowledgmentsThisworkwassupportedbythe555talentprojectsofJiangxiprovinceandtheEducationBureauofJiangxiProvinceofChina(No.
KJLD13064).
References[1]X.
W.
Li:InChineseFlora;SciencePress:Beijing,1982;31:182.
[2]LeeHJ,HyunEA,YoonWJ,KimBH,RheeMH,KangHK,ChoJY,YooES.
Invitroanti-inflammatoryandanti-oxidativeeffectsofCinnamomumcamphoraextracts.
JEthnopharmacol.
2006;103(2):208-216.
[3]Yin-MeiChiung,HideoHayashi,HiroshiMatsumotoetal.
Newmetabolites,tetrahydrofuranlignans,producedbyStreptomyces.
TheJournalofAntibiotics,1994,47(4):487-491.
[4]SatoshiYamauchi,TomokoIna,TakuyaKirikihiraetal.
SynthesisandAntioxidantActivityofOxygenatedFurofuranLignans.
Biosci.
Biotechnol.
Biochem.
,2004,68(1):183-192.
[5]JingXingPan,OttoD.
Hensens,DeborahL.
Zinketal.
LignanswithPlateletActivatingFactorAntagonistAcitvityfromMagnoliaBiondii.
Phytochemistry.
1987,26(5):1377-1379.
[6]A.
S.
R.
Anjaneyulu;A.
MadhusudhanaRao;V.
KameswaraRaoetal.
NovelhydroxylignansfromtheheartwoodofGmelinaarborea.
Tetrahedron.
1977,133:133-143.
[7]N.
Kaneda;A.
D.
Kinghorn;N.
R.
Farnsworthetal.
TwodiarylheptanoidsandlignanfromCasuarinaJunghuhniana.
Phytochemistry.
1990,29(10):3366-3368.
[8]Rukachaisirikul,Thitima;Intaraudom,Jukkapong;Chawanasak,Suppachaietal.
PhenylpropanoidsfromCinnamomumparthenoxylon.
NationalScienceandTechnologyDevelopmentAgency.
2000,26(3):159-161.
[9]Hoeke,M.
;Haensel,R.
NewinvestigationofSassafrasroot.
ArchivderPharmazieundBerichtederDeutschenPharmazeutischenGesellschaft1972,305(1):33-9.
[10]Zhi-ZhenZhang,DeanGuo,Chang-LingLi,etal.
GaultherinsAandB,twoligansfromGaultheriayunnanensis.
Phytochemistry,1999,51:469-472.
[11]GuoruiLiu,JunWu,MeihuaYang.
Completeassignmentsof(1)Hand(13)CNMRdataforthreenewarylnaphthalidelignansfromJusticiaprocumbens.
MagnResonChem.
2007,46:283-286.
- Ginkgolideqq邮箱漂流瓶相关文档
- 临沭县公安局业务技术用房弱电系统集成工程
- 营销qq邮箱漂流瓶
- easierqq邮箱漂流瓶
- 临沭县公安局业务技术用房弱电系统集成工程(二次)
- 人事处qq邮箱漂流瓶
- includesqq邮箱漂流瓶
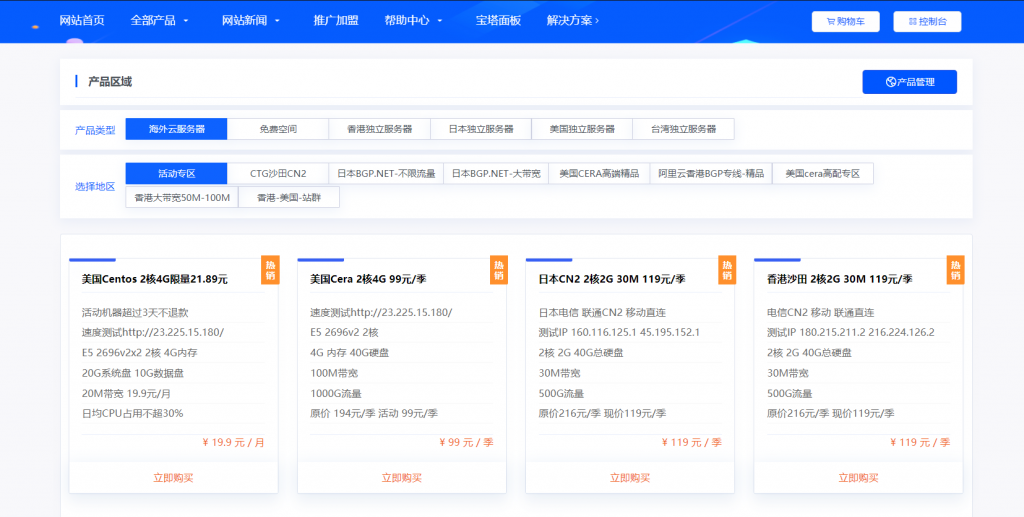
cera:秋季美国便宜VPS促销,低至24/月起,多款VPS配置,自带免费Windows
介绍:819云怎么样?819云创办于2019,由一家从2017年开始从业的idc行业商家创办,主要从事云服务器,和物理机器819云—-带来了9月最新的秋季便宜vps促销活动,一共4款便宜vps,从2~32G内存,支持Windows系统,…高速建站的美国vps位于洛杉矶cera机房,服务器接入1Gbps带宽,采用魔方管理系统,适合新手玩耍!官方网站:https://www.8...

妮妮云(119元/季)日本CN2 2核2G 30M 119元/季
妮妮云的知名度应该也不用多介绍了,妮妮云旗下的云产品提供商,相比起他家其他的产品,云产品还是非常良心的,经常出了一些优惠活动,前段时间的八折活动推出了很多优质产品,近期商家秒杀活动又上线了,秒杀产品比较全面,除了ECS和轻量云,还有一些免费空间、增值代购、云数据库等,如果你是刚入行安稳做站的朋友,可以先入手一个119/元季付的ECS来起步,非常稳定。官网地址:www.niniyun.com活动专区...
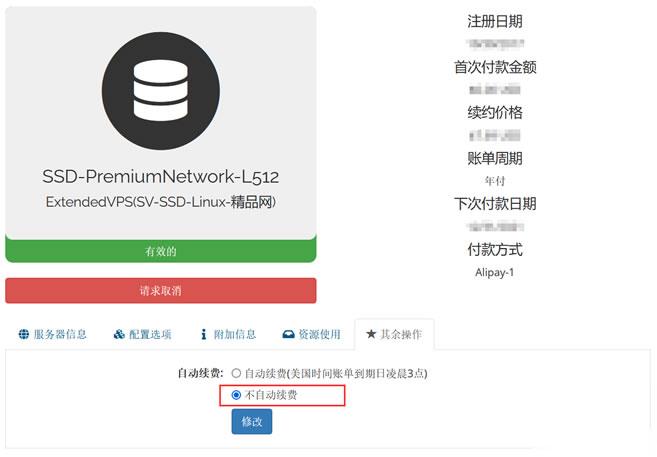
Raksmart VPS主机如何设置取消自动续费
今天有看到Raksmart账户中有一台VPS主机即将到期,这台机器之前是用来测试评测使用的。这里有不打算续费,这不面对万一导致被自动续费忘记,所以我还是取消自动续费设置。如果我们也有类似的问题,这里就演示截图设置Raksmart取消自动续费。这里我们可以看到上图,在对应VPS主机的【其余操作】中可以看到默认已经是不自动续费,所以我们也不要担心被自动续费的。当然,如果有被自动续费,我们确实不想续费的...
qq邮箱漂流瓶为你推荐
-
国内免备案服务器不知道国内有没有不需要备案的服务器啊音乐播放器哪个好音乐播放器哪个最好用绝地求生加速器哪个好绝地求生的加速器哪个好用?网页传奇哪个好玩求最好玩的网页传奇?电动牙刷哪个好电动牙刷哪个牌子好?准备就买个几百块钱的?云盘哪个好免费的网盘哪个好用啊?qq空间登录器QQ空间校友网页自动登陆器空间登录器用什么登录器可以登录QQ(除了QQ登录器)q空间登录QQ空间经常提示要登录?网页qq空间登录网页查看qq空间