sciencepcpie
pcpie 时间:2021-01-27 阅读:()
STUDYPROTOCOLOpenAccessDevelopmentofaninternationallyagreedminimaldatasetforjuveniledermatomyositis(JDM)forclinicalandresearchuseLizaJ.
McCann1*,JamieJ.
Kirkham2,LucyR.
Wedderburn3,4,5,ClarissaPilkington4,5,AdamM.
Huber6,AngeloRavelli7,DuncanAppelbe2,PaulaR.
Williamson2andMichaelW.
Beresford1,8AbstractBackground:Juveniledermatomyositis(JDM)isarareautoimmuneinflammatorydisorderassociatedwithsignificantmorbidityandmortality.
Internationalcollaborationisnecessarytobetterunderstandthepathogenesisofthedisease,responsetotreatmentandlong-termoutcome.
Toaidinternationalcollaboration,itisessentialtohaveacoresetofdatathatallresearchersandclinicianscollectinastandardisedwayforclinicalpurposesandforresearch.
Thisshouldincludedemographicdetails,diagnosticdataandmeasuresofdiseaseactivity,investigationsandtreatment.
VariablesinexistingclinicalregistrieshavebeencomparedtoproduceaprovisionaldatasetforJDM.
Wenowaimtodevelopthisintoaconsensus-approvedminimumcoredataset,testedinawidersetting,withtheobjectiveofachievinginternationalagreement.
Methods/Design:Atwo-stagebespokeDelphi-processwillengagetheopinionofalargenumberofkeystakeholdersthroughEmaildistributionviaestablishedinternationalpaediatricrheumatologyandmyositisorganisations.
This,togetherwithaformalisedpatient/parentparticipationprocesswillhelpinformaconsensusmeetingofinternationalexpertsthatwillutiliseanominalgrouptechnique(NGT).
Theresultingproposedminimaldatasetwillbetestedforfeasibilitywithinexistingdatabaseinfrastructures.
Thedevelopedminimaldatasetwillbesenttoallinternationallyrepresentativecollaboratorsforfinalcomment.
Theparticipantsoftheexpertconsensusgroupwillbeaskedtodrawtogetherthesecomments,ratifyand'signoff'thefinalminimaldataset.
Discussion:Aninternationallyagreedminimaldatasethasthepotentialtosignificantlyenhancecollaboration,alloweffectivecommunicationbetweengroups,provideaminimalstandardofcareandenableanalysisofthelargestpossiblenumberofJDMpatientstoprovideagreaterunderstandingofthisdisease.
Thefinalapprovedminimumcoredatasetcouldberapidlyincorporatedintonationalandinternationalcollaborativeefforts,includingexistingprospectivedatabases,andbeavailableforuseinrandomisedcontrolledtrialsandfortreatment/protocolcomparisonsincohortstudies.
Keywords:Juvenile,Childhood,Dermatomyositis,Idiopathicinflammatorymyopathy,International,Collaboration,Dataset,Coreoutcomeset,Coredataset,Diseaseregister,Clinicaltrials,Diseaseactivity*Correspondence:liza.
mccann@alderhey.
nhs.
uk1AlderHeyChildren'sNHSFoundationTrust,EatonRoad,Liverpool,UKFulllistofauthorinformationisavailableattheendofthearticleTRIALS2015McCannetal.
ThisisanOpenAccessarticledistributedunderthetermsoftheCreativeCommonsAttributionLicense(http://creativecommons.
org/licenses/by/4.
0),whichpermitsunrestricteduse,distribution,andreproductioninanymedium,providedtheoriginalworkisproperlycredited.
TheCreativeCommonsPublicDomainDedicationwaiver(http://creativecommons.
org/publicdomain/zero/1.
0/)appliestothedatamadeavailableinthisarticle,unlessotherwisestated.
McCannetal.
Trials(2015)16:268DOI10.
1186/s13063-015-0784-0BackgroundItisrecognisedthataninternationallyagreedcoredata-setforuseinroutineclinicalcarecouldhavenumerouspotentialbenefitsforpatientswiththisraredisease,withtheadditionaladvantageofinformingfutureresearch[1].
Thegoalsofthisstudyaretoreachinternationalconsensusonaminimaldataset(clinical,laboratoryandpatient/parentreportedmeasures)forpatientswithju-veniledermatomyositis(JDM),whicharepractical,butcoverthekeyvariablesthatallowaccurateassessmentofdiseaseactivityandwouldmeasurechange,suchasre-sponsetotreatment.
Suchacoresetcouldthenberapidlyincorporatedintonationalandinternationalcollaborativeefforts.
Todate,2distinctbutoverlappingcoresetsforJDMhavebeendevelopedforuseinclinicaltrialsbytheInternationalMyositisandClinicalStudies(IMACS)group[2–4]andthePaediatricRheumatologyInter-nationalTrialsOrganisation(PRINTO)[5–7].
Theseex-cellenttoolshavebeenprincipallydesignedforresearchandcanbedifficulttouseinroutineclinicalpracticeduetotimeconstraints.
Ouraimistocomplimentthesecoresetswithaseparateinternationallyagreedminimaldatasetforuseinclinicalpractice.
Thekeyachievableobjectiveofthisstudyisforthedevelopedminimaldatasettoberecognisedandacceptedinternationallytoallowdatacol-lectionovertimeonsufficientnumbersofJDMpatients.
Inordertoachievethis,aformalconsensus-drivenmeth-odologyisrequiredofallstakeholders.
Backgroundworktothisproposalisdescribedelsewhere[1].
Inbrief,aworkinggroupoffiveJDMexperts(CP,LW,AH,AR,LM)fromtheUK,ItalyandCanada,repre-sentativeofthemajorgroupsstudyingJDMandmaintain-ingdatabases,scrutinisedclinicalandlaboratoryvariablescontainedwithincurrentnationalandinternationalcol-laborativedatabases.
Variablescontainedwithin2ormoredatabaseswereconsideredforinclusionintheprovisionalminimaldataset,informedbyaliteraturesearchandde-tailedanalysisoftheUKJuvenileDermatomyositisCohortandBiomarkerStudy(JDCBS)[1,8].
DetailsofthisprojecthavebeenincludedintheCoreOutcomeMeasuresinEffectivenessTrials(COMET)ini-tiativedatabase[9,10].
InadditiontoclosecollaborationwithCOMET,ourgrouphassoughtadvicefromseniormembersoftheOMERACT(OutcomeMeasuresinRheumatology)collaboration,whoarerenownedfortheirworkrelatingtooutcomestandardisation[11,12].
Methods/DesignAprovisionaldatasetforJDM,developedthroughafor-malprocess[1]hasbeenusedasatemplatetoaidastructuredmulti-stageconsensusprocess.
TheDelphitechnique[13–15]hasbeenusedtogaintheopinionoflargegroupsofinternationallyrepresentativeclinicianscaringforchildrenandyoungpeoplewithJDM.
This,togetherwithastepwiseapproachtogaintheopinionofpatients/parents,willhelpinformadetailedface-to-facenominalgroupconsensusprocessbyinternationallyrep-resentativeJDMexperts.
Stage1a–DelphiprocesstogainclinicianopinionA2-stagesurveytechnique(Delphi1andDelphi2;witheachstagebuildingontheproceedingone)hasbeenadopted,sothatanonymousresponsesareobtainedwithequalinfluencetoallindividualparticipants[13–15].
Aninitiallistofallpotentialvariableswasobtainedfromtheprovisionalminimaldatabaseproducedbythisgroup[1]withoutcomeslistedindividuallybutgroupedundertherelevantdomaintoaidinterpretationbyclinicians.
Out-comesidentifiedaspatient/parentreportedoutcomemeasuresorvalidatedtoolsofmusclestrengthmeasure-ment[16]werepresentedwithintheappropriatedomainasuseofthetoolratherthanasindividualcomponentsofthetool.
However,inordertoavoiddiscussionsatthisstagearoundwhichtool/instrumentisbetterformeas-urementasopposedtoanothertool,aninitialquestionaskedparticipantshowimportanttheyratetheuseofatool(withoutspecifyingwhichtool)tomeasureacertaindomain,suchasmusclestrengthorskininvolvement.
Iftheyconsideredatooltobeimportant,theywereaskedwhichtooltheyfavoured.
Thelistofvariableswasreviewedandapprovedbyallauthorsaswellastheresearchsteeringcommitteeorleadingrepresentativesofeachoftheprincipalpartnerorganisations(detailedinTable1),includingtheInter-nationalMyositisandClinicalStudiesgroup–IMACS[4],ChildhoodArthritisandRheumatologyResearchAl-liance–CARRA[17],JuvenileDermatomyositisRe-searchGroup–JDRG(UKandIreland),[18],PaediatricRheumatologyEuropeanSociety–PReSJDMworkingparty[19],andthePaediatricRheumatologyINter-nationalTrialsOrganisation–PRINTO[7].
Aftermodi-ficationsaddressingfeedbackfromthesegroups,thelist(showninTable2)wasformattedintoabespokeelec-tronicquestionnaire.
TheUKPaediatricRheumatologyTrainees'GroupandindividualsfromoutsidetheUKse-lectedbymembersoftheJDMMinimalDatasetSteeringCommittee,pilotedthequestionnairetodetermineaveragetimetakentocompleteandreadabilityofthequestions.
ParticipantdetailsParticipationfortheDelphisurveyswasinvitedviamembershipofIMACS,CARRA,JDRG,PReSJDMworkingpartyandPRINTO;representativeofinter-nationalpaediatricrheumatologyandmyositisspecialtygroups.
Theseorganisationsincludeclinicians(clinicalacademicandnon-academic),scientistsandalliedhealthprofessionalswithexpertiseinpaediatricrheumatologicalconditionsandjuvenile-onsetandadult-onsetmyositisMcCannetal.
Trials(2015)16:268Page2of15Table1DetailsofcollaborativegroupsandpartnerorganisationsCollaborativeorganisationMembershipMaingeographicalareacoveredCollaborativeroleinstudyJuvenileDermatomyositisResearchGroup,JDRG(UKandIreland)[18]Multi-disciplinaryhealthcareprofessionalsandscientistsinthefieldofPaediatricRheumatology;representativeofallmajorUKpaediatricrheumatologycentres.
JDRGmembersareallcontributorsto,andinvestigatorsin,theJuvenileDermatomyositisCohortandBiomarkerStudy,JDCBS[8]UKandIrelandDelphisurveyEmaildistributionRepresentationinNGTmeetingScrutinisingdatacollection(Stage3)AiddisseminationofstudyresultsSteeringcommittee–studyoversightPatient/parentrepresentation:steeringcommitteeandfacilitatedgroupsChildhoodArthritisandRheumatologyResearchAlliance,CARRA[17]PaediatricrheumatologistsandresearchersthroughoutNorthAmerica(n>390);aproportionofwhomhaveaninterestinJDMandothersmoreexperiencedinotherareasofpaediatricrheumatologyNorthAmericaDelphisurveysEmaildistributionRepresentationinNGTmeetingandprovisionalminimaldatasetAiddisseminationofstudyresultsPaediatricRheumatologyINternationalTrialsOrganisation,PRINTO[7]InternationalresearchnetworkwiththeaimofcoordinatingclinicaltrialsandoutcomestudiesinpaediatricrheumatologyInitiallyaEuropeancollaborationbutnowincludes>50countriesand>350centresworldwideDelphisurveysviaE-maildistributionRepresentationinNGTmeetingandprovisionalminimaldatasetAiddisseminationofstudyresultsPaediatricRheumatologyEuropeanSociety,PReS[19]Europeanscientificsocietyforhealthcareprofessionalsinthefieldofpaediatricrheumatology.
ThePReSJDMworkinggroupisasub-groupofthisorganisationthatinvitesindividualswithaninterestorexpertiseinJDMAllEuropeancountries(EUandnon-EU),extendedtotheMiddleEast.
AssociatememberswelcomeworldwideDelphisurveysviaEmaildistributionRepresentationinNGTmeetingAiddisseminationofstudyresultsInternationalMyositisAssessmentandClinicalStudiesgroup,IMACS[4]Coalitionofhealthcareprovidersandresearcherswithaninterestinmyositissyndromes.
MyositissyndromesincludeJDMbutalsootherinflammatorymyopathiesthataremorecommoninadultpatientsPartoftheNIHscienceresearchprogramme,USAbutwelcomesmembersgloballyDelphisurveysviaEmaildistributionRepresentationinNGTmeetingAiddisseminationofstudyresultsEuromyositis[39,40]Europeaninitiativeleadingtothecreationofaweb-basedregistryforadultmyositispatients,recentlyexpandedtoincludepaediatricsInitiallyEuropeancounties(EUandnon-EU)expandedtoincludeothercollaboratorsoutsideEurope(Japan,Mexico,China,US)CollaboratorsinformationofaminimaldatasetRepresentationinNGTmeetingTestingdatacollectionovertime(Stage3,potential)OMERACT[11]AnindependentinitiativeofinternationalhealthprofessionalsinterestedinoutcomemeasuresinrheumatologyNorthAmerica,EuropeandAsia-PacificAdvisoryroleindevelopmentofDelphisurveyandinpatientinvolvementRepresentationinNGTmeetingJDM,juveniledermatomyositis;NGT,nominalgrouptechnique;NIH,NationalInstituteforHealthMcCannetal.
Trials(2015)16:268Page3of15Table2SummaryofquestionsaskedincliniciansurveycomparedtotheparentandpatientquestionnairesDomainQuestionsaskedofhealthcareprofessionalsinDelphi1and2QuestionsaskedinparentquestionnaireQuestionsaskedinyoungperson'squestionnaireGeneralformatofquestionnaireTwoseparatesections:SectionA–whenpatientseenfortheirfirstvisitonly;SectionB–allvisits(firstandsubsequent).
Askedtorateimportanceofeachvariableona1–9scaleseparatelyforclinicaluseandforresearchAskedtorate:HowimportantdoyouthinkeachoneoftheseiswhenthinkingabouthowwellorunwellyourchildisduetoJDM("notthatimportant"/"important"/or"reallyimportant").
Notaskedseparatelyaboutclinical/researchAskedtorate:HowimportantdoyouthinkeachoneoftheseiswhenthinkingabouthowwellorunwellyouareduetoJDM("notthatimportant"/"important"/"reallyimportant")Notaskedseparatelyaboutclinical/researchGeneralquestionsoncollectingandstoringinformationand/orspecimensN/AAskedtoratehowimportanttheythinkitisforalldoctorsandnurses/therapiststocollectkeyinformationinthesamewaywhentheyarelookingafterchildrenwithjuveniledermatomyositis(JDM)Askedtoratehowimportanttheythinkitisforalldoctorsandnurses/therapiststocollectkeyinformationinthesamewaywhentheyarelookingafterchildrenwithJDMHowimportantdoyouratestoringspecimensatdiagnosisforotherbiomarkers(eg.
,DNA/serum/anyothermaterial)aAskedtoratehowimportanttheythinkitistostorethetypeofinformationcollectedinclinicinaresearchdatabaseAskedtoratehowimportanttheythinkitistostorethetypeofinformationcollectedinclinicinaresearchdatabaseDemographicsofpatientDateofbirthN/AN/AGenderRaceEthnicityFamilyhistoryOfautoimmunediseaseN/AN/AOfneuromusculardiseaseDiagnosticdata(general)AgeofonsetofJDMN/AN/AAgeofdiagnosisofJDMMUSCLE–Diagnostic/activitydataPresenceofsymmetricalproximalmuscleweakness:atdiagnosis(sectionA)andeveryvisit(sectionB)AskingyouifyourchildhasanyweaknessAskingifyouhaveanyweaknessPresenceofothermuscleweakness(eg.
,distalorasymmetrical):sectionBonlyUseofavalidatedtooltoscoremusclestrengthand/orfunction(optionsfordifferentmusclestrengthscores)aTestingtoseehowstrongyourchildisTestingtoseehowstrongyouareSKIN–Diagnostic/activitydataHeliotroperash(sectionsAandB)AskingyouifyourchildhasanyskinrashesAskingifyouhaveanyskinrashesGottron'spapules/Gottron'ssign(sectionsAandB)Nail-foldcapillarychanges(sectionsAandB)OthercharacteristicJDMrashUseofavalidatedskintoolforJDM(optionsgiventochoosespecifictools)aLookingforrashesorskinsignsthatmaysuggestactiveJDMLookingforrashesorskinsignsthatmaysuggestactiveJDMSKIN–AdditionalactivitydataaskedinsectionBonlyLipodystrophyN/AN/ACalcinosisCutaneousulcerationMcCannetal.
Trials(2015)16:268Page4of15Table2Summaryofquestionsaskedincliniciansurveycomparedtotheparentandpatientquestionnaires(Continued)SubcutaneousoedemaMalar/facialerythemaShawlsign/V-signMechanic'shandsAlopeciaVasculopathiclesionsPhotosensitivityLividoreticularisOthererythemaPanniculitisMAJORORGANINVOLVEMENTduetomyositis–askedinsectionBonlyofcliniciansurvey,pluspatient/parentquestionnairesMusculoskeletalinvolvement(specificquestionsaddedforarthritis/contractures)aAskingifyourchildhasanymuscleorjointpainsAskingifyouhaveanymuscleorjointpainsLookingforswellinginanyofthejointsLookingforswellinginanyofthejointsGastrointestinalinvolvement(specificquestionsaddedfordysphagia/abdominalpainorGIulceration)aAskingifyourchildhasanydifficultywithswallowing/eatingoriftheyhavetummypainAskingifyouhaveanydifficultywitheatingorifyouhavetummypainPulmonaryinvolvementsuggestinginterstitiallungdiseaseAskingifyourchildhasanyshortnessofbreathorchestpainthatmayberelatedtoJDMAskingifyouhaveanyshortnessofbreathorchestpainthatmayberelatedtoJDMCardiacinvolvementCONSTITUTIONALSYMPTOMSduetomyositis–askedinsectionBonlyofcliniciansurveyandinpatient/parentquestionnaireFever(>38°C)duetomyositisN/AN/AWeightloss(>5%)duetomyositisN/AN/AFatigueduetomyositisAskingifyourchildfeelstiredduetoJDMAskingifyoufeeltiredduetoJDMAskinghowtiredyourchildfeelsusingaformal"fatiguescale"Askinghowtiredyoufeelusingaformal'fatiguescale'IrritabilityduetomyositisAskingifyourchildfeelsirritableormiserableduetoJDMAskingifyoufeelirritableormiserableduetoJDMRaynaud'sphenomenonAskingifyourchildgetscolourchangesintheirhandsincoldweatherAskingifyougetcolourchangesinyourhandsincoldweatherGrowthHeightofpatient(sectionBonly)Checkinghowwellyourchildisgrowing(height)CheckinghowwellyouaregrowingWeightofpatient(sectionBonly)Askingaboutanyweightlossandcheckingyourchild'sweightAskingaboutanyweightlossandcheckingyourweightDevelopment/pubertyN/AAskingabouthowyourchildisdevelopingorhowpubertyisprogressingAskingabouthowyouaredevelopingorhowpubertyisprogressingOtherinformationPresenceofmalignancy(sectionBonly)N/AN/APatienthavingongoingfollow-upatthiscentre(Ifno:optionsgivenforreasonwhy)aGlobaldiseaseactivityAskingyourdoctortomarkona0–10cmscalehowwellorunwelltheythinkyourchildhasbeenAskingyourdoctortomarkona0–10cmscalehowwellorunwelltheythinkyouhavebeenMcCannetal.
Trials(2015)16:268Page5of15Table2Summaryofquestionsaskedincliniciansurveycomparedtotheparentandpatientquestionnaires(Continued)Useofaphysician-scoredmeasureofglobaldiseaseactivity-suchasphysicianVisualAnalogueScale;VAS(specificchoicesforactivityscoresgiven)abasedonwhatyoutellthemandwhattheyseeatyourclinicvisitPatient/parentReportedOutcomeMeasures(PROMs),QualityofLife/SchoolissuesUseofapatient/parentreportedoutcomemeasureofdiseaseactivityoruseofavalidatedmeasureoffunction(suchaspatientVAS/CHAQ)and/oratooltomeasureQualityofLife(QoL)(specificchoicesgiven)aAskingyouoryourchildtocompleteaquestionnairethatlooksathoweasyordifficultitisforthemtodothingslikegetdressed,haveabath,doactivitiesAskingyouortocompletealistofquestionsthatlookathoweasyordifficultitisforyoutodothingslikegetdressed,haveabath,doactivitiesAskingyou/yourchildtomarkona0–10cmscalehowwellorunwellyourchildhasbeenoverthelast4weeksAskingyoutomarkona0–10cmscalehowwellorunwellyouhavebeenoverthelast4weeksduetoJDMAskingyou/yourchildtomarkona0–-10cmscalehowmuchpainyourchildhashadinthelast4weeksduetoJDMAskingyoutomarkona0–10cmscalehowmuchpainyouhavehadinthelast4weeksduetoJDMAskinghowmanydaysyourchildhasmissedschoolorcollegeduetoJDMAskinghowmanydaysyouhavemissedschoolorcollegeduetoJDMAskingmorequestionsaboutyourchild'sschool–howarethingsAretheyabletokeepupwithpeersAskingmorequestionsaboutschool–howarethingsAreyouabletokeepupwithyourpeersAskingyourchildhowtheyfeelemotionallyinrelationtotheirJDM(questionsrelatingtoQualityofLifeormood)AskingaboutyourfeelingsinrelationtoyourJDMINVESTIGATIONS:diagnosticdata/diseaseactivitydataElevationofmuscleenzymesatdiagnosis(sectionA)orlaterindiseasecourse(sectionB).
(Questionsaskedaboutwhichspecificenzymestomeasure)aTakingbloodteststomonitorhowactivethediseaseisTakingbloodteststomonitorhowactivethediseaseisElectromyography(EMG)changesofmyositisatdiagnosis(sectionA)Musclebiopsyevidenceofmyositisatdiagnosis(sectionA)MagneticResonanceImaging(MRI)changesofmyositisatdiagnosis(sectionA)AskingyourchildtohaveascansuchasanMRIscanoftheirmusclestomonitorthediseaseAskingyoutohaveascansuchasanMRIscanofyourmusclesSectionBonly:abnormalinvestigations(imaging,histologyorcardio-pulmonaryfunctiontestsindicatingflareofmyositis).
If"yes,"tickwhichinvestigationsareabnormalAnti-nuclearAntibody(ANA)positivityatdiagnosis(sectionAonly)Myositisspecificantibody(MSA)positiveatdiagnosis(sectionAonly)Myositisassociatedantibody(MAA)positivityatdiagnosis(sectionAonly)TreatmentDatetreatmentstarted(mm/yyyy)Askingspecificquestionsaboutyourchild'smedicinesandhowtheymakethemfeelAnyunwantedeffectsAskingspecificquestionsaboutyourmedicinesandhowtheymakeyoufeelAnyunwantedeffectsMcCannetal.
Trials(2015)16:268Page6of15Table2Summaryofquestionsaskedincliniciansurveycomparedtotheparentandpatientquestionnaires(Continued)PatienttakingsteroidsYes/No(optionsfortypeofsteroidgiven)aAskingyouaboutthemedicinesyourchildistakingatthemomentAskingyouaboutthemedicinesyouaretakingatthemomentPatienttakingaDiseaseModifyingAnti-RheumaticDrug(DMARD)(NotincludingbiologicDMARDS)Yes/No(withoptiontoselectnameofdrugfromalist)PatienttakingabiologicYes/No(withoptiontoselectnameofdrugfromalist)Patienthavingphysiotherapyand/oroccupationaltherapyAskingyouifyourchildisdoinganyphysiotherapyexercisesAskingyouifyouaredoingyourphysiotherapyexercisesOtherquestionsTodeterminepracticeandexperience-primaryrole,patientgroup,numberofpaediatric/adultpatientsundertheircare,geographicalregionofpracticeandmembershipofsocieties/professionalbodiesHowwouldyoupreferinformationtobeaskedofyou(eg.
,inclinic,questionnairebeforeclinic,questionnairebeforeyoucometoclinic)Howwouldyoupreferinformationtobeaskedofyou(eg.
,inclinic,questionnairebeforeclinic,questionnairebeforeyoucometoclinic)FurtherinformationArethereanyvariablesthatyouthinkareimportantforclinicalcarethatarenotcurrentlyincludedinthelistaboveIfso,pleasestatewhattheseare(samequestionalsoaskedseparatelyforvariablesimportantforresearch)Arethereanyotherkeyquestionsthatwehavemissedbutthatyouthinkitisimportantforustoaskwhenyoucometoclinic,thinkingabouthowwell/unwellyou/yourchildisIfso,whatkeyquestionsaretheyArethereanyotherkeyquestionsthatwehavemissedbutthatyouthinkareimportantforustoaskwhenyoucometoclinic,thinkingabouthowwellorunwellyouareIfso,whatkeyquestionsaretheyIsthereanythinginthislist(orfromyourexperienceofbringingyourchildtoclinic)thatyoudonotthinkshouldbeincludedoryouthinkyourchilddoesnotlikeIfso,whatWhydoyouthinkthatthesequestionsshouldnotbeincludedIsthereanythinginthislist(orfromyourexperienceofcomingtoclinic)thatyoudonotlikeIfso,whatWhydoyouthinkthatthesequestionsshouldnotbeincludedaFurtherdetailedquestionsaskedinhiddenrows,exposediftheanswertotheprecedingquestionsuggeststhattheparticipantthinksthatthevariableisimportantN/A,notapplicableMcCannetal.
Trials(2015)16:268Page7of15(Table1).
TheentiremembershipwascontactedbyEmailandinvitedtoparticipateinthetwoweb-basedquestion-nairesusingabespokeDelphisystem.
TheonlyexceptiontothiswasthePRINTOmembership.
InviewofthefactthatPRINTOisalargeandheterogeneousorganisationwithover900individualmembers,thePRINTOdirectors(approximately400members)werecontacted.
PRINTOdirectorsrepresentallofthemajorpaediatricrheumatol-ogycentresamongmembercountries.
ManyEmailrecipi-entsarepartofmorethanoneresearchorganisationandinthiscase,theywereaskedtocompleteeachsurveyroundonceonlyandidentifywhichothergroupstheyaremembersof(and,therefore,potentiallypartofmorethanonemailing).
Delphiround1Thestudyobjectiveswereclearlystatedincludingwhatwasexpectedofeachparticipant.
ParticipantswereremindedoftheimportanceofcompletingtheentireDel-phiprocessandaskedtocompleteeachroundwithin6weeksofreceiptofEmail.
ReminderEmailsweresentonafortnightlybasistoaidcompletionofeachround,withphrasingofremindersconsideredtomaximisethere-sponserates[20].
Participantswhoagreedtotakepartwereaskedtoregister,allowingauniqueidentifiertobeallocatedtoenabletrackingofattritionateachroundandidentificationoftheresearchgroupthattheparticipantwasrespondingfrom(IMACS,CARRA,PRINTO,PReSJDMworkinggrouportheUKJDRG).
Contributorswereaskedtospecifytheirpredominantclinicalrole(clinician/scientist/alliedhealthprofessional/trainee),theirspecialty(specialistormajorinterestinrheumatology/neurology/dermatology/immunology/other),theageofpatientthattheypredominantlycarefor(adult/paediatric)theirexperi-enceinthespecialty/condition(≥or16yearsofage)whohaveorpreviouslyhadjuvenile-onsetmyositisandparentsofchildrenwithJDM.
NHSResearchEthicsapprovalobtainedStage2(nominalgroupconsensus)doesnotinvolvepatientdataandsimplyinvolvesconsensusamongexpertsinmyositisStage3willinvolveanalysisofanonymiseddataafterpilotingtheminimaldatasetinaproportionofpatientsoverarestrictedperiodoftime(6months)viaalreadyMcCannetal.
Trials(2015)16:268Page12of15establisheddatabases.
Ethicalapprovalforthisstageiscountryspecific,andalthoughitwillinvolveanonymisedpatientdata,itisrequiredforsomecollaborators.
EthicalapprovalisalreadyobtainedfordatacollectionwithinmostoftheorganisationsthatmaycollaborateatthisstageincludingtheUKJDCBS,Euromyositis,theGasliniInstituteandCARRA.
OtherparticipantswillbeabletoenterdataviaEuromyositisorobtaintheirownIRBapproval.
However,thestudyisnotdependentonIRBapprovaloftheseindividualcountries,assufficientpatientnumbersshouldbeachievedviauseofestablisheddatabasesthatalreadyincludeethicalapproval.
Ourgroupwillonlyhaveaccess,throughresearchsteeringcommitteeapplication,tosecondaryanonymiseddatacollectedwithintheseindividualdatabases.
DiscussionTheDelphiprocesshastheadvantageofallowinginfor-mationtobeexchangedbetweennumerousindividualswhomaybegeographicallydispersed[38].
However,theadvantageofface-to-faceinteractionisthatitallowsidentificationofthereasonsfordisagreementsbetweenindividuals[38].
ByusingacombinationofDelphiweb-basedsurveysandface-to-faceinteractioninanNGTconsensusgroupprocess,thisstudywillobtainwide-spreadopinionfromalargenumberofcliniciansinadditiontothejudgmentofasmallgroupofinter-nationallyrenownedandrepresentativegroupofexpertsinJDM.
Patientandparentparticipationwillbeensuredviaastructuredprocess,includingwidespreaddistribu-tionofaquestionnaireandmoredetaileddiscussionwithinestablishedpatient/parentgroupsintheUK.
Theuseoffocusgroupswillallowstimulationofdiscussionandcomparisonofexperiencesacrossparticipants,whereasindividualquestionnaireresponseswillallowobtainmentofopinionswithoutinfluencefrompeers[30,41].
Inaddition,considerationwillbegiventoasmallnumberofpatient/parentrepresentativespartici-patingintheconsensusmeeting,representingtheviewsofthisgroup.
Aninternationallyagreedminimaldatasethasthepo-tentialtosignificantlyenhancecollaboration,allowef-fectivecommunicationbetweengroups,provideaminimalstandardofcareandenableanalysisofthelar-gestpossiblenumberofJDMpatientstoprovideagreaterunderstandingofthisdisease.
Aconsensus-driven,internationallyapprovedminimumcoredatasetcouldberapidlyincorporatedintonationalandinter-nationalcollaborativeefforts,includingexistingpro-spectiveclinicaldatabases,andbeavailableforuseinrandomisedcontrolledtrialsandfortreatment/protocolcomparisonsincohortstudies.
StudystatusCurrentlyatStage3:Delphisurveyofcliniciansandconsensusmeetingcompleted.
Patient/parentquestion-nairesongoing.
AbbreviationsCRN:ClinicalResearchNetwork;CSG:ClinicalStudiesGroup;BSPAR:BritishSocietyforPaediatricandAdolescentRheumatology;CARRA:ChildhoodArthritisandRheumatologyResearchAlliance;COMET:CoreOutcomeMeasuresinEffectivenessTrialsinitiative;GCP:GoodClinicalPractice;GRADE:GradeofRecommendationsAssessment,DevelopmentandEvaluation;JDCBS:JuvenileDermatomyositisCohortBiomarkerStudyandRepository(UKandIreland);JDM:juveniledermatomyositis;JDRG:JuvenileDermatomyositisResearchGroup(UKandIreland);IMACS:InternationalMyositisandClinicalStudiesgroup;MCRN:MedicinesforChildrenResearchNetwork;NIHR:NationalInstituteforHealthResearch;NGT:nominalgrouptechnique;OMERACT:OutcomeMeasuresinRheumatology;PReS:PaediatricRheumatologyEuropeanSociety;PRINTO:PaediatricRheumatologyINternationalTrialsOrganisation;SHARE:SingleHubandAccessPointforPaediatricRheumatologyinEurope;TSG:TopicSpecificGroup.
CompetinginterestsTheauthorsdeclarethattheyhavenocompetinginterests.
Authors'contributionAllauthorshavebeendirectlyinvolvedindevelopingthemethodologyforthisstudy.
LMhasledallpartsofthestudyincludingbackgroundwork,preparationoftheprotocol,ethicssubmissions,contentofDelphisurveyandpatient/parentquestionnaires,planningofconsensusmeetingandwritingofthemanuscript.
JKparticipatedinthedesignofthestudy,wasresponsiblefortestingtheDelphisystem,performedthestatisticalanalysisandhelpedpreparefortheconsensusmeeting.
LW,CP,AHandARhaveprovidedintellectualinputandpracticalhelpintoallpartsofthestudyincludingbackgroundwork,protocoldevelopment,Delphisurveyandplanningoftheconsensusmeeting.
DAdevelopedthebespokeDelphisystemandprovidedITsupportforthestudyincludingdataanalysis.
PWhasprovidedexpertadviceonthestudymethodologyandanalysis.
MBhasbeenresponsibleforintellectualandfinancialoverviewofthestudyandinputintotheprotocoldevelopment,Delphisurveyandconsensusmeeting.
Allauthorshavereadandapprovedthefinalmanuscript.
Authors'informationDrLizaMcCannisaconsultantinpaediatricrheumatologyatAlderHeyChildren'sNHSFoundationTrust,UK,witharesearchinterestinjuveniledermatomyositis.
DrJamieKirkhamisalecturerinbiostatisticsattheUniversityofLiverpool,InstituteofTranslationalMedicine,UK.
ProfessorLucyWedderburnisProfessorofPaediatricRheumatologyattheInstituteofChildHealth,UniversityCollegeLondon;consultantatGreatOrmondStreetHospital;ChiefInvestigatoroftheJuvenileDermatomyositisCohortandBiomarkerStudy,(JDCBS)UKandIrelandandDirectorofArthritisResearchUKCentreforAdolescentRheumatologyatUCL.
DrClarissaPilkingtonisaconsultantinpaediatricandadolescentrheumatologyatGreatOrmondStreetandUniversityCollegeHospital,London,UK;PresidentoftheBritishSocietyforPaediatricandAdolescentRheumatology.
Shehasaspecialinterestinjuveniledermatomyositisandsystemiclupuserythematosis.
DrAdamHuberisapaediatricrheumatologistattheIWKHealthCentreandAssociateProfessoratDalhousieUniversity,withaparticularresearchinterestinjuvenilemyositis.
ProfessorAngeloRavelliisAssociateProfessorofPaediatricsattheUniversityofGenoaandIstitutoGianninaGaslini,Genoa,Italy.
Hisresearchinterestsincludejuvenilemyositis.
DrDuncanAppelbeistheInformationSystemsManagerattheMCRNClinicalTrialsUnit,UniversityofLiverpoolInstituteofTranslationalMedicine,UK.
ProfessorPaulaWilliamsonisHeadofDepartmentandDirectoroftheClinicalTrialsResearchCentreattheUniversityofLiverpoolInstituteofTranslationalMedicine.
McCannetal.
Trials(2015)16:268Page13of15ProfessorMichaelWBeresfordisBroughChairandProfessorofChildHealth,UniversityofLiverpool;AcademicLeadintheClinicalAcademicDepartmentofPaediatricRheumatologyatAlderHeyChildren'sNHSFoundationTrust,LiverpoolUKandChairoftheUK'sNationalInstituteofHealthResearchClinicalResearchNetwork:Children/ArthritisResearchUKPaediatricRheumatologyClinicalStudiesGroup.
AcknowledgmentsThisworkwassupportedbyArthritisResearchUK[grantnumber20417].
WewouldliketoacknowledgetheEuromyositisResearchSteeringCommitteefortheircollaborationandimpetusforthiswork,inparticularProfessorIngridLundberg,DrHectorChinoy,ProfessorJiriVencovskyandNielsSteenKrogh.
WeacknowledgetheIMACS,CARRA,PRINTOandPReSworkinggroupsfortheirsupportandcollaboration.
Inparticular,wewouldliketoacknowledgethefollowingcollaboratorswhorepresentthesegroups:DrLisaRider,ProfessorCharlesSpencer,DrNicolaRupertoandDrAnnetvanRoyen-Kerkhof.
WeacknowledgethesupportofOMERACTandinparticular,wouldliketothankProfessorMaartenBoersforhisadvisoryrole.
WewouldliketoacknowledgeourcollaboratorswithintheCOMETgroup,inparticular,HeatherBagley,PatientandPublicInvolvementCoordinator.
WewouldalsoliketoacknowledgethehelpandadviceofProfessorBridgetYoung,OliviaLloydandHelenHansonandtheClinicalStudiesGroupconsumerrepresentativesandBSPARParentGroup,particularlySharonDouglas.
WewouldliketothankyoungpeoplefromNIHRYoungPerson'sAdvisoryGroupandtheJDMYoungPerson'sGroup,fortheiradviceonpatientquestionnairesandinformationleaflets.
Weacknowledgethesupportandcollaborationofpatient/parentsupportgroups,CureJMandMyositisUK.
WewouldliketothankKatieArnold,LawrenceBrown,KathForrestandKarenBarnesfortheiradministrativesupportforthiswork.
TheUKJDMCohortandBiomarkerstudyhasbeensupportedbygenerousgrantsfromtheWellcomeTrustUK(085860),ActionMedicalResearchUK,(SP4252),TheMyositisSupportGroupUK,ArthritisResearchUK(14518),TheHenrySmithCharity.
LWsworkissupportedinpartbyGreatOrmondStreetChildren'sCharity,theGOSH/ICHNIHRfundedBiomedicalresearchCentre(BRC)andArthritisResearchUK.
TheJDMCohortstudyisadoptedontotheComprehensiveResearchNetworkthroughtheMedicinesforChildrenResearchNetwork(www.
mcrn.
org.
uk)andissupportedbytheGOSH/ICHBiomedicalResearchCentre.
AfulllistofcontributorstotheJDCBScanbefoundontheJDRGwebsitehttp://www.
juveniledermatomyositis.
org.
uk.
Authordetails1AlderHeyChildren'sNHSFoundationTrust,EatonRoad,Liverpool,UK.
2MRCNorthWestHubforTrialsMethodologyResearch,DepartmentofBiostatistics,UniversityofLiverpool,Liverpool,UK.
3Infection,Immunology,andRheumatologySectionUCLInstituteofChildHealth,UniversityCollegeLondon,London,UK.
4GreatOrmondStreetHospitalNHSFoundationTrust,London,UK.
5CentreforAdolescentRheumatologyatUniversityCollegeLondon,UniversityCollegeLondonHospital,London,UK.
6IWKHealthCentreandDalhousieUniversity,5850UniversityAvenue,Halifax,NSB3K6R8,Canada.
7UniversitàdegliStudidiGenovaandIstitutoGianninaGaslini,ViaG.
Gaslini5,16147Genoa,Italy.
8DepartmentofWomen'sandChildren'sHealth,InstituteofTranslationalMedicine,UniversityofLiverpool,Liverpool,UK.
Received:19February2015Accepted:29May2015References1.
McCannLJ,ArnoldK,PilkingtonCA,HuberAM,RavelliA,BeardL,etal.
Developingaprovisional,internationalMinimalDatasetforJuvenileDermatomyositis:foruseinclinicalpracticetoinformresearch.
Pediatr.
Rheumatol.
2014;12:31.
2.
RiderLG,GianniniEH,Harris-LoveM,JoeG,IsenbergD,PilkingtonC,etal.
Definingclinicalimprovementinadultandjuvenilemyositis.
JRheumatol.
2003;30:603–17.
3.
RiderLG,GianniniEH,BrunnerHI,RupertoN,James-NewtonL,ReedAM,etal.
Internationalconsensusonpreliminarydefinitionsofimprovementinadultandjuvenilemyositis.
ArthritisRheum.
2004;50:2281–90.
4.
InternationalMyositisAssessmentandClinicalStudiesGroup,IMACS.
http://www.
niehs.
nih.
gov/research/resources/imacs5.
RupertoN,RavelliA,PistorioA,FerrianiV,CalvoI,GanserG,etal.
TheprovisionalPaediatricRheumatologyInternationalTrialsOrganisation/AmericanCollegeofRheumatology/EuropeanLeagueAgainstRheumatismDiseaseactivitycoresetfortheevaluationofresponsetotherapyinjuveniledermatomyositis:aprospectivevalidationstudy.
ArthritisRheum.
2008;59:4–13.
6.
RupertoN,PistorioA,RavelliA,RiderLG,PilkingtonC,OliveiraS,etal.
ThePaediatricRheumatologyInternationalTrialsOrganisationprovisionalcriteriafortheevaluationofresponsetotherapyinjuveniledermatomyositis.
ArthritisCareRes(Hoboken).
2010;62:1533–41.
7.
PaediatricRheumatologyINternationalTrialsOrganisation,PRINTO.
http://www.
printo.
it/.
8.
MartinN,KrolP,SmithS,MurrayK,PilkingtonCA,DavidsonJE,etal.
Anationalregistryforjuveniledermatomyositisandotherpaediatricidiopathicinflammatorymyopathies:10years'experience;theJuvenileDermatomyositisNational(UKandIreland)CohortBiomarkerStudyandRepositoryforIdiopathicInflammatoryMyopathies.
Rheumatology(Oxford).
2011;50:137–45.
9.
CoreOutcomeMeasuresinEffectivenessTrials(COMET)Initiativedatabasehttp://www.
cometinitiative.
org.
10.
GargonE,WilliamsonPR,AltmanDG,BlazebyJM,ClarkeM.
TheCOMETInitiativedatabase:progressandactivitiesfrom2011–2013.
Trials.
2014;15:279.
11.
OMERACT(OutcomeMeasuresinRheumatology)collaboration.
http://www.
omeract.
org/.
12.
BoersM,KirwanJR,WellsG,BeatonD,GossecL,d'AgostinoMA,etal.
DevelopingCoreOutcomeMeasurementSetsforclinicaltrials:OMERACTFilter2.
0.
JClinEpidemiol.
2013;67(7):745–53.
13.
SinhaIP,SmythRL,WilliamsonPR.
UsingtheDelphitechniquetodeterminewhichoutcomestomeasureinclinicaltrials:recommendationsforthefuturebasedonasystematicreviewofexistingstudies.
PLoSMed.
2011;8(1),e1000393.
14.
JonesJ,HunterD.
Qualitativeresearch:consensusmethodsformedicalandhealthservicesresearch.
BMJ.
1995;311(7001):376–80.
15.
CantrillJA,SibbaldB,BuetowS.
TheDelphiandnominalgrouptechniquesinhealthservicesresearch.
IntJPharmPract.
1996;4(2):67–74.
16.
RiderLG,WerthVP,HuberAM,AlexandersonH,RaoAP,RupertoN,etal.
Measuresofadultandjuveniledermatomyositis,polymyositis,andinclusionbodymyositis:PhysicianandPatient/ParentGlobalActivity,ManualMuscleTesting(MMT),HealthAssessmentQuestionnaire(HAQ)/ChildhoodHealthAssessmentQuestionnaire(C-HAQ),ChildhoodMyositisAssessmentScale(CMAS),MyositisDiseaseActivityAssessmentTool(MDAAT),DiseaseActivityScore(DAS),ShortForm36(SF-36),ChildHealthQuestionnaire(CHQ),physicianglobaldamage,MyositisDamageIndex(MDI),QuantitativeMuscleTesting(QMT),MyositisFunctionalIndex-2(FI-2),MyositisActivitiesProfile(MAP),InclusionBodyMyositisFunctionalRatingScale(IBMFRS),CutaneousDermatomyositisDiseaseAreaandSeverityIndex(CDASI),CutaneousAssessmentTool(CAT),DermatomyositisSkinSeverityIndex(DSSI),Skindex,andDermatologyLifeQualityIndex(DLQI).
ArthritisCareRes(Hoboken).
2011;63Suppl11:S118–57.
17.
ChildhoodArthritisandRheumatologyResearchAlliance,CARRA.
www.
carragroup.
org.
18.
JuvenileDermatomyositisResearchGroupUKandIreland,JDRG.
http://www.
juveniledermatomyositis.
org.
uk.
19.
PaediatricRheumatologyEuropeanSociety,PReS.
http://www.
pres.
org.
uk/.
20.
DillmanDA,SmytheJD,ChristianLM.
Mailandinternetsurveys:thetailoreddesignmethod.
2nded.
Hoboken,NJ:Wiley;2007.
21.
GuyattGH,OxmanAD,KunzR,AtkinsD,BrozekJ,VistG,etal.
GRADEguidelines:2Framingthequestionanddecidingonimportantoutcomes.
JClinEpidemiol.
2011;64(4):395–400.
22.
RiderLG,KoziolD,GianniniEH,JainMS,SmithMR,Whitney-MahoneyK,etal.
Validationofmanualmuscletestingandasubsetofeightmuscles(MMT8)foradultandjuvenileidiopathicinflammatorymyopathies.
ArthritisCareRes.
2010;62(4):465–72.
23.
StringerE,BohnsackJ,BowyerSL,GriffinTA,HuberAM,LangB,etal.
Treatmentapproachestojuveniledermatomyositis(JDM)acrossNorthAmerica:TheChildhoodArthritisandRheumatologyResearchAlliance(CARRA)JDMTreatmentSurvey.
JRheumatol.
2010;37(9):1953–61.
24.
BrownVE,PilkingtonCA,FeldmanBM,DavidsonJE,onbehalfoftheNetworkforJuvenileDermatomyositis,aworkingpartyofthePaediatricRheumatologyEuropeanSociety(PReS).
Aninternationalconsensussurveyofthediagnosticcriteriaforjuveniledermatomyositis(JDM).
Rheumatology.
2006;45:990–3.
25.
BrunnerHI,MinaR,PilkingtonC,BeresfordMW,ReiffA,LevyDM,etal.
Preliminarycriteriaforglobalflaresinchildhood-onsetsystemiclupuserythematosus.
ArthritisCareRes(Hoboken).
2011;63(9):1213–23.
McCannetal.
Trials(2015)16:268Page14of1526.
RupertoN,HanrahanLM,AlarcónGS,BelmontHM,BreyRL,BrunettaP,etal.
Internationalconsensusforadefinitionofdiseaseflareinlupus.
Lupus.
2011;20(5):453–62.
27.
NIHRCRN:Children/ArthritisResearchUKPaediatricRheumatologyClinicalStudiesGroup.
http://www.
arthritisresearchuk.
org/research/our-clinical-study-groups-and-research-strategies/paediatric-rheumatology.
aspx.
28.
MyositisUK.
http://www.
myositis.
org.
uk/.
29.
CureJMFoundation.
http://www.
curejm.
org/.
30.
MatzaLS,PatrickDL,RileyAW,AlexanderJJ,RajmilL,PleilAM,etal.
Pediatricpatient-reportedoutcomeinstrumentsforresearchtosupportmedicalproductlabeling:reportoftheISPORPROgoodresearchpracticesfortheassessmentofchildrenandadolescentstaskforce.
ValueHealth.
2013;16(4):461–79.
31.
SMOG(simplifiedmeasureofgobbledygook)grading.
http://www.
niace.
org.
uk/misc/SMOG-calculator/smogcalc.
php.
32.
TheBritishSocietyforPaediatricandAdolescentRheumatology(BSPAR).
http://www.
bspar.
org.
uk/.
33.
NationalInstituteforHealthResearch(NIHR)ClinicalResearchNetwork(CRN)YoungPerson'sAdvisoryGroup.
http://www.
crn.
nihr.
ac.
uk/children/pcpie/young-persons-advisory-group/.
34.
RupertoN,RavelliA,MurrayKJ,LovellDJ,Andersson-GareB,FeldmanBM,etal.
Preliminarycoresetsofmeasuresfordiseaseactivityanddamageassessmentinjuvenilesystemiclupuserythematosusandjuveniledermatomyositis.
Rheumatology.
2003;42:1452–9.
35.
NugentJ,RupertoN,GraingerJ,MachadoC,SawhneyS,BaildamE,etal.
TheBritishversionoftheChildhoodHealthAssessmentQuestionnaire(CHAQ)andtheChildHealthQuestionnaire(CHQ).
ClinExpRheumatol.
2001;2001(19):S163–7.
36.
VarnierGC,FerrariC,ConsolaroA,MarafonD,PilkingtonC,MaillardS,etal.
Introducinganewapproachtothecareofjuveniledermatomyositis:thejuveniledermatomyositismultidimensionalassessmentreport.
PediatrRheumato.
2013;11Suppl2:25.
37.
WulffraatNM,VastertB,theSHAREconsortium.
Timetoshare.
PediatrRheumatol.
2013;11(5)http://www.
ped-rheum.
com/content/11/1/5.
38.
MurphyMK,BlackNA,LampingDL,McKeeCM,SandersonCF,AskhamJ,MarteauT.
Consensusdevelopmentmethods,andtheiruseinclinicalguidelinedevelopment:areview.
HealthTechnologyAssessment.
1998.
2(3).
39.
Euromyositis.
http://Euromyositis.
eu40.
LundbergIE,SvenssonJ.
Registriesinidiopathicinflammatorymyopathies.
CurrOpinRheumatol.
2013;25:729–34.
41.
PatrickDL,BurkeLB,GwaltneyCJ,LeidyNK,MartinML,MolsenE,etal.
Contentvalidity–establishingandreportingtheevidenceinnewlydevelopedPatient-ReportedOutcomes(PRO)instrumentsformedicalproductevaluation:ISPORPROGoodResearchPracticesTaskForceReport:Part1–ElicitingconceptsforanewPROinstrument.
ValueHealth.
2011;14:967–77.
SubmityournextmanuscripttoBioMedCentralandtakefulladvantageof:ConvenientonlinesubmissionThoroughpeerreviewNospaceconstraintsorcolorgurechargesImmediatepublicationonacceptanceInclusioninPubMed,CAS,ScopusandGoogleScholarResearchwhichisfreelyavailableforredistributionSubmityourmanuscriptatwww.
biomedcentral.
com/submitMcCannetal.
Trials(2015)16:268Page15of15
McCann1*,JamieJ.
Kirkham2,LucyR.
Wedderburn3,4,5,ClarissaPilkington4,5,AdamM.
Huber6,AngeloRavelli7,DuncanAppelbe2,PaulaR.
Williamson2andMichaelW.
Beresford1,8AbstractBackground:Juveniledermatomyositis(JDM)isarareautoimmuneinflammatorydisorderassociatedwithsignificantmorbidityandmortality.
Internationalcollaborationisnecessarytobetterunderstandthepathogenesisofthedisease,responsetotreatmentandlong-termoutcome.
Toaidinternationalcollaboration,itisessentialtohaveacoresetofdatathatallresearchersandclinicianscollectinastandardisedwayforclinicalpurposesandforresearch.
Thisshouldincludedemographicdetails,diagnosticdataandmeasuresofdiseaseactivity,investigationsandtreatment.
VariablesinexistingclinicalregistrieshavebeencomparedtoproduceaprovisionaldatasetforJDM.
Wenowaimtodevelopthisintoaconsensus-approvedminimumcoredataset,testedinawidersetting,withtheobjectiveofachievinginternationalagreement.
Methods/Design:Atwo-stagebespokeDelphi-processwillengagetheopinionofalargenumberofkeystakeholdersthroughEmaildistributionviaestablishedinternationalpaediatricrheumatologyandmyositisorganisations.
This,togetherwithaformalisedpatient/parentparticipationprocesswillhelpinformaconsensusmeetingofinternationalexpertsthatwillutiliseanominalgrouptechnique(NGT).
Theresultingproposedminimaldatasetwillbetestedforfeasibilitywithinexistingdatabaseinfrastructures.
Thedevelopedminimaldatasetwillbesenttoallinternationallyrepresentativecollaboratorsforfinalcomment.
Theparticipantsoftheexpertconsensusgroupwillbeaskedtodrawtogetherthesecomments,ratifyand'signoff'thefinalminimaldataset.
Discussion:Aninternationallyagreedminimaldatasethasthepotentialtosignificantlyenhancecollaboration,alloweffectivecommunicationbetweengroups,provideaminimalstandardofcareandenableanalysisofthelargestpossiblenumberofJDMpatientstoprovideagreaterunderstandingofthisdisease.
Thefinalapprovedminimumcoredatasetcouldberapidlyincorporatedintonationalandinternationalcollaborativeefforts,includingexistingprospectivedatabases,andbeavailableforuseinrandomisedcontrolledtrialsandfortreatment/protocolcomparisonsincohortstudies.
Keywords:Juvenile,Childhood,Dermatomyositis,Idiopathicinflammatorymyopathy,International,Collaboration,Dataset,Coreoutcomeset,Coredataset,Diseaseregister,Clinicaltrials,Diseaseactivity*Correspondence:liza.
mccann@alderhey.
nhs.
uk1AlderHeyChildren'sNHSFoundationTrust,EatonRoad,Liverpool,UKFulllistofauthorinformationisavailableattheendofthearticleTRIALS2015McCannetal.
ThisisanOpenAccessarticledistributedunderthetermsoftheCreativeCommonsAttributionLicense(http://creativecommons.
org/licenses/by/4.
0),whichpermitsunrestricteduse,distribution,andreproductioninanymedium,providedtheoriginalworkisproperlycredited.
TheCreativeCommonsPublicDomainDedicationwaiver(http://creativecommons.
org/publicdomain/zero/1.
0/)appliestothedatamadeavailableinthisarticle,unlessotherwisestated.
McCannetal.
Trials(2015)16:268DOI10.
1186/s13063-015-0784-0BackgroundItisrecognisedthataninternationallyagreedcoredata-setforuseinroutineclinicalcarecouldhavenumerouspotentialbenefitsforpatientswiththisraredisease,withtheadditionaladvantageofinformingfutureresearch[1].
Thegoalsofthisstudyaretoreachinternationalconsensusonaminimaldataset(clinical,laboratoryandpatient/parentreportedmeasures)forpatientswithju-veniledermatomyositis(JDM),whicharepractical,butcoverthekeyvariablesthatallowaccurateassessmentofdiseaseactivityandwouldmeasurechange,suchasre-sponsetotreatment.
Suchacoresetcouldthenberapidlyincorporatedintonationalandinternationalcollaborativeefforts.
Todate,2distinctbutoverlappingcoresetsforJDMhavebeendevelopedforuseinclinicaltrialsbytheInternationalMyositisandClinicalStudies(IMACS)group[2–4]andthePaediatricRheumatologyInter-nationalTrialsOrganisation(PRINTO)[5–7].
Theseex-cellenttoolshavebeenprincipallydesignedforresearchandcanbedifficulttouseinroutineclinicalpracticeduetotimeconstraints.
Ouraimistocomplimentthesecoresetswithaseparateinternationallyagreedminimaldatasetforuseinclinicalpractice.
Thekeyachievableobjectiveofthisstudyisforthedevelopedminimaldatasettoberecognisedandacceptedinternationallytoallowdatacol-lectionovertimeonsufficientnumbersofJDMpatients.
Inordertoachievethis,aformalconsensus-drivenmeth-odologyisrequiredofallstakeholders.
Backgroundworktothisproposalisdescribedelsewhere[1].
Inbrief,aworkinggroupoffiveJDMexperts(CP,LW,AH,AR,LM)fromtheUK,ItalyandCanada,repre-sentativeofthemajorgroupsstudyingJDMandmaintain-ingdatabases,scrutinisedclinicalandlaboratoryvariablescontainedwithincurrentnationalandinternationalcol-laborativedatabases.
Variablescontainedwithin2ormoredatabaseswereconsideredforinclusionintheprovisionalminimaldataset,informedbyaliteraturesearchandde-tailedanalysisoftheUKJuvenileDermatomyositisCohortandBiomarkerStudy(JDCBS)[1,8].
DetailsofthisprojecthavebeenincludedintheCoreOutcomeMeasuresinEffectivenessTrials(COMET)ini-tiativedatabase[9,10].
InadditiontoclosecollaborationwithCOMET,ourgrouphassoughtadvicefromseniormembersoftheOMERACT(OutcomeMeasuresinRheumatology)collaboration,whoarerenownedfortheirworkrelatingtooutcomestandardisation[11,12].
Methods/DesignAprovisionaldatasetforJDM,developedthroughafor-malprocess[1]hasbeenusedasatemplatetoaidastructuredmulti-stageconsensusprocess.
TheDelphitechnique[13–15]hasbeenusedtogaintheopinionoflargegroupsofinternationallyrepresentativeclinicianscaringforchildrenandyoungpeoplewithJDM.
This,togetherwithastepwiseapproachtogaintheopinionofpatients/parents,willhelpinformadetailedface-to-facenominalgroupconsensusprocessbyinternationallyrep-resentativeJDMexperts.
Stage1a–DelphiprocesstogainclinicianopinionA2-stagesurveytechnique(Delphi1andDelphi2;witheachstagebuildingontheproceedingone)hasbeenadopted,sothatanonymousresponsesareobtainedwithequalinfluencetoallindividualparticipants[13–15].
Aninitiallistofallpotentialvariableswasobtainedfromtheprovisionalminimaldatabaseproducedbythisgroup[1]withoutcomeslistedindividuallybutgroupedundertherelevantdomaintoaidinterpretationbyclinicians.
Out-comesidentifiedaspatient/parentreportedoutcomemeasuresorvalidatedtoolsofmusclestrengthmeasure-ment[16]werepresentedwithintheappropriatedomainasuseofthetoolratherthanasindividualcomponentsofthetool.
However,inordertoavoiddiscussionsatthisstagearoundwhichtool/instrumentisbetterformeas-urementasopposedtoanothertool,aninitialquestionaskedparticipantshowimportanttheyratetheuseofatool(withoutspecifyingwhichtool)tomeasureacertaindomain,suchasmusclestrengthorskininvolvement.
Iftheyconsideredatooltobeimportant,theywereaskedwhichtooltheyfavoured.
Thelistofvariableswasreviewedandapprovedbyallauthorsaswellastheresearchsteeringcommitteeorleadingrepresentativesofeachoftheprincipalpartnerorganisations(detailedinTable1),includingtheInter-nationalMyositisandClinicalStudiesgroup–IMACS[4],ChildhoodArthritisandRheumatologyResearchAl-liance–CARRA[17],JuvenileDermatomyositisRe-searchGroup–JDRG(UKandIreland),[18],PaediatricRheumatologyEuropeanSociety–PReSJDMworkingparty[19],andthePaediatricRheumatologyINter-nationalTrialsOrganisation–PRINTO[7].
Aftermodi-ficationsaddressingfeedbackfromthesegroups,thelist(showninTable2)wasformattedintoabespokeelec-tronicquestionnaire.
TheUKPaediatricRheumatologyTrainees'GroupandindividualsfromoutsidetheUKse-lectedbymembersoftheJDMMinimalDatasetSteeringCommittee,pilotedthequestionnairetodetermineaveragetimetakentocompleteandreadabilityofthequestions.
ParticipantdetailsParticipationfortheDelphisurveyswasinvitedviamembershipofIMACS,CARRA,JDRG,PReSJDMworkingpartyandPRINTO;representativeofinter-nationalpaediatricrheumatologyandmyositisspecialtygroups.
Theseorganisationsincludeclinicians(clinicalacademicandnon-academic),scientistsandalliedhealthprofessionalswithexpertiseinpaediatricrheumatologicalconditionsandjuvenile-onsetandadult-onsetmyositisMcCannetal.
Trials(2015)16:268Page2of15Table1DetailsofcollaborativegroupsandpartnerorganisationsCollaborativeorganisationMembershipMaingeographicalareacoveredCollaborativeroleinstudyJuvenileDermatomyositisResearchGroup,JDRG(UKandIreland)[18]Multi-disciplinaryhealthcareprofessionalsandscientistsinthefieldofPaediatricRheumatology;representativeofallmajorUKpaediatricrheumatologycentres.
JDRGmembersareallcontributorsto,andinvestigatorsin,theJuvenileDermatomyositisCohortandBiomarkerStudy,JDCBS[8]UKandIrelandDelphisurveyEmaildistributionRepresentationinNGTmeetingScrutinisingdatacollection(Stage3)AiddisseminationofstudyresultsSteeringcommittee–studyoversightPatient/parentrepresentation:steeringcommitteeandfacilitatedgroupsChildhoodArthritisandRheumatologyResearchAlliance,CARRA[17]PaediatricrheumatologistsandresearchersthroughoutNorthAmerica(n>390);aproportionofwhomhaveaninterestinJDMandothersmoreexperiencedinotherareasofpaediatricrheumatologyNorthAmericaDelphisurveysEmaildistributionRepresentationinNGTmeetingandprovisionalminimaldatasetAiddisseminationofstudyresultsPaediatricRheumatologyINternationalTrialsOrganisation,PRINTO[7]InternationalresearchnetworkwiththeaimofcoordinatingclinicaltrialsandoutcomestudiesinpaediatricrheumatologyInitiallyaEuropeancollaborationbutnowincludes>50countriesand>350centresworldwideDelphisurveysviaE-maildistributionRepresentationinNGTmeetingandprovisionalminimaldatasetAiddisseminationofstudyresultsPaediatricRheumatologyEuropeanSociety,PReS[19]Europeanscientificsocietyforhealthcareprofessionalsinthefieldofpaediatricrheumatology.
ThePReSJDMworkinggroupisasub-groupofthisorganisationthatinvitesindividualswithaninterestorexpertiseinJDMAllEuropeancountries(EUandnon-EU),extendedtotheMiddleEast.
AssociatememberswelcomeworldwideDelphisurveysviaEmaildistributionRepresentationinNGTmeetingAiddisseminationofstudyresultsInternationalMyositisAssessmentandClinicalStudiesgroup,IMACS[4]Coalitionofhealthcareprovidersandresearcherswithaninterestinmyositissyndromes.
MyositissyndromesincludeJDMbutalsootherinflammatorymyopathiesthataremorecommoninadultpatientsPartoftheNIHscienceresearchprogramme,USAbutwelcomesmembersgloballyDelphisurveysviaEmaildistributionRepresentationinNGTmeetingAiddisseminationofstudyresultsEuromyositis[39,40]Europeaninitiativeleadingtothecreationofaweb-basedregistryforadultmyositispatients,recentlyexpandedtoincludepaediatricsInitiallyEuropeancounties(EUandnon-EU)expandedtoincludeothercollaboratorsoutsideEurope(Japan,Mexico,China,US)CollaboratorsinformationofaminimaldatasetRepresentationinNGTmeetingTestingdatacollectionovertime(Stage3,potential)OMERACT[11]AnindependentinitiativeofinternationalhealthprofessionalsinterestedinoutcomemeasuresinrheumatologyNorthAmerica,EuropeandAsia-PacificAdvisoryroleindevelopmentofDelphisurveyandinpatientinvolvementRepresentationinNGTmeetingJDM,juveniledermatomyositis;NGT,nominalgrouptechnique;NIH,NationalInstituteforHealthMcCannetal.
Trials(2015)16:268Page3of15Table2SummaryofquestionsaskedincliniciansurveycomparedtotheparentandpatientquestionnairesDomainQuestionsaskedofhealthcareprofessionalsinDelphi1and2QuestionsaskedinparentquestionnaireQuestionsaskedinyoungperson'squestionnaireGeneralformatofquestionnaireTwoseparatesections:SectionA–whenpatientseenfortheirfirstvisitonly;SectionB–allvisits(firstandsubsequent).
Askedtorateimportanceofeachvariableona1–9scaleseparatelyforclinicaluseandforresearchAskedtorate:HowimportantdoyouthinkeachoneoftheseiswhenthinkingabouthowwellorunwellyourchildisduetoJDM("notthatimportant"/"important"/or"reallyimportant").
Notaskedseparatelyaboutclinical/researchAskedtorate:HowimportantdoyouthinkeachoneoftheseiswhenthinkingabouthowwellorunwellyouareduetoJDM("notthatimportant"/"important"/"reallyimportant")Notaskedseparatelyaboutclinical/researchGeneralquestionsoncollectingandstoringinformationand/orspecimensN/AAskedtoratehowimportanttheythinkitisforalldoctorsandnurses/therapiststocollectkeyinformationinthesamewaywhentheyarelookingafterchildrenwithjuveniledermatomyositis(JDM)Askedtoratehowimportanttheythinkitisforalldoctorsandnurses/therapiststocollectkeyinformationinthesamewaywhentheyarelookingafterchildrenwithJDMHowimportantdoyouratestoringspecimensatdiagnosisforotherbiomarkers(eg.
,DNA/serum/anyothermaterial)aAskedtoratehowimportanttheythinkitistostorethetypeofinformationcollectedinclinicinaresearchdatabaseAskedtoratehowimportanttheythinkitistostorethetypeofinformationcollectedinclinicinaresearchdatabaseDemographicsofpatientDateofbirthN/AN/AGenderRaceEthnicityFamilyhistoryOfautoimmunediseaseN/AN/AOfneuromusculardiseaseDiagnosticdata(general)AgeofonsetofJDMN/AN/AAgeofdiagnosisofJDMMUSCLE–Diagnostic/activitydataPresenceofsymmetricalproximalmuscleweakness:atdiagnosis(sectionA)andeveryvisit(sectionB)AskingyouifyourchildhasanyweaknessAskingifyouhaveanyweaknessPresenceofothermuscleweakness(eg.
,distalorasymmetrical):sectionBonlyUseofavalidatedtooltoscoremusclestrengthand/orfunction(optionsfordifferentmusclestrengthscores)aTestingtoseehowstrongyourchildisTestingtoseehowstrongyouareSKIN–Diagnostic/activitydataHeliotroperash(sectionsAandB)AskingyouifyourchildhasanyskinrashesAskingifyouhaveanyskinrashesGottron'spapules/Gottron'ssign(sectionsAandB)Nail-foldcapillarychanges(sectionsAandB)OthercharacteristicJDMrashUseofavalidatedskintoolforJDM(optionsgiventochoosespecifictools)aLookingforrashesorskinsignsthatmaysuggestactiveJDMLookingforrashesorskinsignsthatmaysuggestactiveJDMSKIN–AdditionalactivitydataaskedinsectionBonlyLipodystrophyN/AN/ACalcinosisCutaneousulcerationMcCannetal.
Trials(2015)16:268Page4of15Table2Summaryofquestionsaskedincliniciansurveycomparedtotheparentandpatientquestionnaires(Continued)SubcutaneousoedemaMalar/facialerythemaShawlsign/V-signMechanic'shandsAlopeciaVasculopathiclesionsPhotosensitivityLividoreticularisOthererythemaPanniculitisMAJORORGANINVOLVEMENTduetomyositis–askedinsectionBonlyofcliniciansurvey,pluspatient/parentquestionnairesMusculoskeletalinvolvement(specificquestionsaddedforarthritis/contractures)aAskingifyourchildhasanymuscleorjointpainsAskingifyouhaveanymuscleorjointpainsLookingforswellinginanyofthejointsLookingforswellinginanyofthejointsGastrointestinalinvolvement(specificquestionsaddedfordysphagia/abdominalpainorGIulceration)aAskingifyourchildhasanydifficultywithswallowing/eatingoriftheyhavetummypainAskingifyouhaveanydifficultywitheatingorifyouhavetummypainPulmonaryinvolvementsuggestinginterstitiallungdiseaseAskingifyourchildhasanyshortnessofbreathorchestpainthatmayberelatedtoJDMAskingifyouhaveanyshortnessofbreathorchestpainthatmayberelatedtoJDMCardiacinvolvementCONSTITUTIONALSYMPTOMSduetomyositis–askedinsectionBonlyofcliniciansurveyandinpatient/parentquestionnaireFever(>38°C)duetomyositisN/AN/AWeightloss(>5%)duetomyositisN/AN/AFatigueduetomyositisAskingifyourchildfeelstiredduetoJDMAskingifyoufeeltiredduetoJDMAskinghowtiredyourchildfeelsusingaformal"fatiguescale"Askinghowtiredyoufeelusingaformal'fatiguescale'IrritabilityduetomyositisAskingifyourchildfeelsirritableormiserableduetoJDMAskingifyoufeelirritableormiserableduetoJDMRaynaud'sphenomenonAskingifyourchildgetscolourchangesintheirhandsincoldweatherAskingifyougetcolourchangesinyourhandsincoldweatherGrowthHeightofpatient(sectionBonly)Checkinghowwellyourchildisgrowing(height)CheckinghowwellyouaregrowingWeightofpatient(sectionBonly)Askingaboutanyweightlossandcheckingyourchild'sweightAskingaboutanyweightlossandcheckingyourweightDevelopment/pubertyN/AAskingabouthowyourchildisdevelopingorhowpubertyisprogressingAskingabouthowyouaredevelopingorhowpubertyisprogressingOtherinformationPresenceofmalignancy(sectionBonly)N/AN/APatienthavingongoingfollow-upatthiscentre(Ifno:optionsgivenforreasonwhy)aGlobaldiseaseactivityAskingyourdoctortomarkona0–10cmscalehowwellorunwelltheythinkyourchildhasbeenAskingyourdoctortomarkona0–10cmscalehowwellorunwelltheythinkyouhavebeenMcCannetal.
Trials(2015)16:268Page5of15Table2Summaryofquestionsaskedincliniciansurveycomparedtotheparentandpatientquestionnaires(Continued)Useofaphysician-scoredmeasureofglobaldiseaseactivity-suchasphysicianVisualAnalogueScale;VAS(specificchoicesforactivityscoresgiven)abasedonwhatyoutellthemandwhattheyseeatyourclinicvisitPatient/parentReportedOutcomeMeasures(PROMs),QualityofLife/SchoolissuesUseofapatient/parentreportedoutcomemeasureofdiseaseactivityoruseofavalidatedmeasureoffunction(suchaspatientVAS/CHAQ)and/oratooltomeasureQualityofLife(QoL)(specificchoicesgiven)aAskingyouoryourchildtocompleteaquestionnairethatlooksathoweasyordifficultitisforthemtodothingslikegetdressed,haveabath,doactivitiesAskingyouortocompletealistofquestionsthatlookathoweasyordifficultitisforyoutodothingslikegetdressed,haveabath,doactivitiesAskingyou/yourchildtomarkona0–10cmscalehowwellorunwellyourchildhasbeenoverthelast4weeksAskingyoutomarkona0–10cmscalehowwellorunwellyouhavebeenoverthelast4weeksduetoJDMAskingyou/yourchildtomarkona0–-10cmscalehowmuchpainyourchildhashadinthelast4weeksduetoJDMAskingyoutomarkona0–10cmscalehowmuchpainyouhavehadinthelast4weeksduetoJDMAskinghowmanydaysyourchildhasmissedschoolorcollegeduetoJDMAskinghowmanydaysyouhavemissedschoolorcollegeduetoJDMAskingmorequestionsaboutyourchild'sschool–howarethingsAretheyabletokeepupwithpeersAskingmorequestionsaboutschool–howarethingsAreyouabletokeepupwithyourpeersAskingyourchildhowtheyfeelemotionallyinrelationtotheirJDM(questionsrelatingtoQualityofLifeormood)AskingaboutyourfeelingsinrelationtoyourJDMINVESTIGATIONS:diagnosticdata/diseaseactivitydataElevationofmuscleenzymesatdiagnosis(sectionA)orlaterindiseasecourse(sectionB).
(Questionsaskedaboutwhichspecificenzymestomeasure)aTakingbloodteststomonitorhowactivethediseaseisTakingbloodteststomonitorhowactivethediseaseisElectromyography(EMG)changesofmyositisatdiagnosis(sectionA)Musclebiopsyevidenceofmyositisatdiagnosis(sectionA)MagneticResonanceImaging(MRI)changesofmyositisatdiagnosis(sectionA)AskingyourchildtohaveascansuchasanMRIscanoftheirmusclestomonitorthediseaseAskingyoutohaveascansuchasanMRIscanofyourmusclesSectionBonly:abnormalinvestigations(imaging,histologyorcardio-pulmonaryfunctiontestsindicatingflareofmyositis).
If"yes,"tickwhichinvestigationsareabnormalAnti-nuclearAntibody(ANA)positivityatdiagnosis(sectionAonly)Myositisspecificantibody(MSA)positiveatdiagnosis(sectionAonly)Myositisassociatedantibody(MAA)positivityatdiagnosis(sectionAonly)TreatmentDatetreatmentstarted(mm/yyyy)Askingspecificquestionsaboutyourchild'smedicinesandhowtheymakethemfeelAnyunwantedeffectsAskingspecificquestionsaboutyourmedicinesandhowtheymakeyoufeelAnyunwantedeffectsMcCannetal.
Trials(2015)16:268Page6of15Table2Summaryofquestionsaskedincliniciansurveycomparedtotheparentandpatientquestionnaires(Continued)PatienttakingsteroidsYes/No(optionsfortypeofsteroidgiven)aAskingyouaboutthemedicinesyourchildistakingatthemomentAskingyouaboutthemedicinesyouaretakingatthemomentPatienttakingaDiseaseModifyingAnti-RheumaticDrug(DMARD)(NotincludingbiologicDMARDS)Yes/No(withoptiontoselectnameofdrugfromalist)PatienttakingabiologicYes/No(withoptiontoselectnameofdrugfromalist)Patienthavingphysiotherapyand/oroccupationaltherapyAskingyouifyourchildisdoinganyphysiotherapyexercisesAskingyouifyouaredoingyourphysiotherapyexercisesOtherquestionsTodeterminepracticeandexperience-primaryrole,patientgroup,numberofpaediatric/adultpatientsundertheircare,geographicalregionofpracticeandmembershipofsocieties/professionalbodiesHowwouldyoupreferinformationtobeaskedofyou(eg.
,inclinic,questionnairebeforeclinic,questionnairebeforeyoucometoclinic)Howwouldyoupreferinformationtobeaskedofyou(eg.
,inclinic,questionnairebeforeclinic,questionnairebeforeyoucometoclinic)FurtherinformationArethereanyvariablesthatyouthinkareimportantforclinicalcarethatarenotcurrentlyincludedinthelistaboveIfso,pleasestatewhattheseare(samequestionalsoaskedseparatelyforvariablesimportantforresearch)Arethereanyotherkeyquestionsthatwehavemissedbutthatyouthinkitisimportantforustoaskwhenyoucometoclinic,thinkingabouthowwell/unwellyou/yourchildisIfso,whatkeyquestionsaretheyArethereanyotherkeyquestionsthatwehavemissedbutthatyouthinkareimportantforustoaskwhenyoucometoclinic,thinkingabouthowwellorunwellyouareIfso,whatkeyquestionsaretheyIsthereanythinginthislist(orfromyourexperienceofbringingyourchildtoclinic)thatyoudonotthinkshouldbeincludedoryouthinkyourchilddoesnotlikeIfso,whatWhydoyouthinkthatthesequestionsshouldnotbeincludedIsthereanythinginthislist(orfromyourexperienceofcomingtoclinic)thatyoudonotlikeIfso,whatWhydoyouthinkthatthesequestionsshouldnotbeincludedaFurtherdetailedquestionsaskedinhiddenrows,exposediftheanswertotheprecedingquestionsuggeststhattheparticipantthinksthatthevariableisimportantN/A,notapplicableMcCannetal.
Trials(2015)16:268Page7of15(Table1).
TheentiremembershipwascontactedbyEmailandinvitedtoparticipateinthetwoweb-basedquestion-nairesusingabespokeDelphisystem.
TheonlyexceptiontothiswasthePRINTOmembership.
InviewofthefactthatPRINTOisalargeandheterogeneousorganisationwithover900individualmembers,thePRINTOdirectors(approximately400members)werecontacted.
PRINTOdirectorsrepresentallofthemajorpaediatricrheumatol-ogycentresamongmembercountries.
ManyEmailrecipi-entsarepartofmorethanoneresearchorganisationandinthiscase,theywereaskedtocompleteeachsurveyroundonceonlyandidentifywhichothergroupstheyaremembersof(and,therefore,potentiallypartofmorethanonemailing).
Delphiround1Thestudyobjectiveswereclearlystatedincludingwhatwasexpectedofeachparticipant.
ParticipantswereremindedoftheimportanceofcompletingtheentireDel-phiprocessandaskedtocompleteeachroundwithin6weeksofreceiptofEmail.
ReminderEmailsweresentonafortnightlybasistoaidcompletionofeachround,withphrasingofremindersconsideredtomaximisethere-sponserates[20].
Participantswhoagreedtotakepartwereaskedtoregister,allowingauniqueidentifiertobeallocatedtoenabletrackingofattritionateachroundandidentificationoftheresearchgroupthattheparticipantwasrespondingfrom(IMACS,CARRA,PRINTO,PReSJDMworkinggrouportheUKJDRG).
Contributorswereaskedtospecifytheirpredominantclinicalrole(clinician/scientist/alliedhealthprofessional/trainee),theirspecialty(specialistormajorinterestinrheumatology/neurology/dermatology/immunology/other),theageofpatientthattheypredominantlycarefor(adult/paediatric)theirexperi-enceinthespecialty/condition(≥or16yearsofage)whohaveorpreviouslyhadjuvenile-onsetmyositisandparentsofchildrenwithJDM.
NHSResearchEthicsapprovalobtainedStage2(nominalgroupconsensus)doesnotinvolvepatientdataandsimplyinvolvesconsensusamongexpertsinmyositisStage3willinvolveanalysisofanonymiseddataafterpilotingtheminimaldatasetinaproportionofpatientsoverarestrictedperiodoftime(6months)viaalreadyMcCannetal.
Trials(2015)16:268Page12of15establisheddatabases.
Ethicalapprovalforthisstageiscountryspecific,andalthoughitwillinvolveanonymisedpatientdata,itisrequiredforsomecollaborators.
EthicalapprovalisalreadyobtainedfordatacollectionwithinmostoftheorganisationsthatmaycollaborateatthisstageincludingtheUKJDCBS,Euromyositis,theGasliniInstituteandCARRA.
OtherparticipantswillbeabletoenterdataviaEuromyositisorobtaintheirownIRBapproval.
However,thestudyisnotdependentonIRBapprovaloftheseindividualcountries,assufficientpatientnumbersshouldbeachievedviauseofestablisheddatabasesthatalreadyincludeethicalapproval.
Ourgroupwillonlyhaveaccess,throughresearchsteeringcommitteeapplication,tosecondaryanonymiseddatacollectedwithintheseindividualdatabases.
DiscussionTheDelphiprocesshastheadvantageofallowinginfor-mationtobeexchangedbetweennumerousindividualswhomaybegeographicallydispersed[38].
However,theadvantageofface-to-faceinteractionisthatitallowsidentificationofthereasonsfordisagreementsbetweenindividuals[38].
ByusingacombinationofDelphiweb-basedsurveysandface-to-faceinteractioninanNGTconsensusgroupprocess,thisstudywillobtainwide-spreadopinionfromalargenumberofcliniciansinadditiontothejudgmentofasmallgroupofinter-nationallyrenownedandrepresentativegroupofexpertsinJDM.
Patientandparentparticipationwillbeensuredviaastructuredprocess,includingwidespreaddistribu-tionofaquestionnaireandmoredetaileddiscussionwithinestablishedpatient/parentgroupsintheUK.
Theuseoffocusgroupswillallowstimulationofdiscussionandcomparisonofexperiencesacrossparticipants,whereasindividualquestionnaireresponseswillallowobtainmentofopinionswithoutinfluencefrompeers[30,41].
Inaddition,considerationwillbegiventoasmallnumberofpatient/parentrepresentativespartici-patingintheconsensusmeeting,representingtheviewsofthisgroup.
Aninternationallyagreedminimaldatasethasthepo-tentialtosignificantlyenhancecollaboration,allowef-fectivecommunicationbetweengroups,provideaminimalstandardofcareandenableanalysisofthelar-gestpossiblenumberofJDMpatientstoprovideagreaterunderstandingofthisdisease.
Aconsensus-driven,internationallyapprovedminimumcoredatasetcouldberapidlyincorporatedintonationalandinter-nationalcollaborativeefforts,includingexistingpro-spectiveclinicaldatabases,andbeavailableforuseinrandomisedcontrolledtrialsandfortreatment/protocolcomparisonsincohortstudies.
StudystatusCurrentlyatStage3:Delphisurveyofcliniciansandconsensusmeetingcompleted.
Patient/parentquestion-nairesongoing.
AbbreviationsCRN:ClinicalResearchNetwork;CSG:ClinicalStudiesGroup;BSPAR:BritishSocietyforPaediatricandAdolescentRheumatology;CARRA:ChildhoodArthritisandRheumatologyResearchAlliance;COMET:CoreOutcomeMeasuresinEffectivenessTrialsinitiative;GCP:GoodClinicalPractice;GRADE:GradeofRecommendationsAssessment,DevelopmentandEvaluation;JDCBS:JuvenileDermatomyositisCohortBiomarkerStudyandRepository(UKandIreland);JDM:juveniledermatomyositis;JDRG:JuvenileDermatomyositisResearchGroup(UKandIreland);IMACS:InternationalMyositisandClinicalStudiesgroup;MCRN:MedicinesforChildrenResearchNetwork;NIHR:NationalInstituteforHealthResearch;NGT:nominalgrouptechnique;OMERACT:OutcomeMeasuresinRheumatology;PReS:PaediatricRheumatologyEuropeanSociety;PRINTO:PaediatricRheumatologyINternationalTrialsOrganisation;SHARE:SingleHubandAccessPointforPaediatricRheumatologyinEurope;TSG:TopicSpecificGroup.
CompetinginterestsTheauthorsdeclarethattheyhavenocompetinginterests.
Authors'contributionAllauthorshavebeendirectlyinvolvedindevelopingthemethodologyforthisstudy.
LMhasledallpartsofthestudyincludingbackgroundwork,preparationoftheprotocol,ethicssubmissions,contentofDelphisurveyandpatient/parentquestionnaires,planningofconsensusmeetingandwritingofthemanuscript.
JKparticipatedinthedesignofthestudy,wasresponsiblefortestingtheDelphisystem,performedthestatisticalanalysisandhelpedpreparefortheconsensusmeeting.
LW,CP,AHandARhaveprovidedintellectualinputandpracticalhelpintoallpartsofthestudyincludingbackgroundwork,protocoldevelopment,Delphisurveyandplanningoftheconsensusmeeting.
DAdevelopedthebespokeDelphisystemandprovidedITsupportforthestudyincludingdataanalysis.
PWhasprovidedexpertadviceonthestudymethodologyandanalysis.
MBhasbeenresponsibleforintellectualandfinancialoverviewofthestudyandinputintotheprotocoldevelopment,Delphisurveyandconsensusmeeting.
Allauthorshavereadandapprovedthefinalmanuscript.
Authors'informationDrLizaMcCannisaconsultantinpaediatricrheumatologyatAlderHeyChildren'sNHSFoundationTrust,UK,witharesearchinterestinjuveniledermatomyositis.
DrJamieKirkhamisalecturerinbiostatisticsattheUniversityofLiverpool,InstituteofTranslationalMedicine,UK.
ProfessorLucyWedderburnisProfessorofPaediatricRheumatologyattheInstituteofChildHealth,UniversityCollegeLondon;consultantatGreatOrmondStreetHospital;ChiefInvestigatoroftheJuvenileDermatomyositisCohortandBiomarkerStudy,(JDCBS)UKandIrelandandDirectorofArthritisResearchUKCentreforAdolescentRheumatologyatUCL.
DrClarissaPilkingtonisaconsultantinpaediatricandadolescentrheumatologyatGreatOrmondStreetandUniversityCollegeHospital,London,UK;PresidentoftheBritishSocietyforPaediatricandAdolescentRheumatology.
Shehasaspecialinterestinjuveniledermatomyositisandsystemiclupuserythematosis.
DrAdamHuberisapaediatricrheumatologistattheIWKHealthCentreandAssociateProfessoratDalhousieUniversity,withaparticularresearchinterestinjuvenilemyositis.
ProfessorAngeloRavelliisAssociateProfessorofPaediatricsattheUniversityofGenoaandIstitutoGianninaGaslini,Genoa,Italy.
Hisresearchinterestsincludejuvenilemyositis.
DrDuncanAppelbeistheInformationSystemsManagerattheMCRNClinicalTrialsUnit,UniversityofLiverpoolInstituteofTranslationalMedicine,UK.
ProfessorPaulaWilliamsonisHeadofDepartmentandDirectoroftheClinicalTrialsResearchCentreattheUniversityofLiverpoolInstituteofTranslationalMedicine.
McCannetal.
Trials(2015)16:268Page13of15ProfessorMichaelWBeresfordisBroughChairandProfessorofChildHealth,UniversityofLiverpool;AcademicLeadintheClinicalAcademicDepartmentofPaediatricRheumatologyatAlderHeyChildren'sNHSFoundationTrust,LiverpoolUKandChairoftheUK'sNationalInstituteofHealthResearchClinicalResearchNetwork:Children/ArthritisResearchUKPaediatricRheumatologyClinicalStudiesGroup.
AcknowledgmentsThisworkwassupportedbyArthritisResearchUK[grantnumber20417].
WewouldliketoacknowledgetheEuromyositisResearchSteeringCommitteefortheircollaborationandimpetusforthiswork,inparticularProfessorIngridLundberg,DrHectorChinoy,ProfessorJiriVencovskyandNielsSteenKrogh.
WeacknowledgetheIMACS,CARRA,PRINTOandPReSworkinggroupsfortheirsupportandcollaboration.
Inparticular,wewouldliketoacknowledgethefollowingcollaboratorswhorepresentthesegroups:DrLisaRider,ProfessorCharlesSpencer,DrNicolaRupertoandDrAnnetvanRoyen-Kerkhof.
WeacknowledgethesupportofOMERACTandinparticular,wouldliketothankProfessorMaartenBoersforhisadvisoryrole.
WewouldliketoacknowledgeourcollaboratorswithintheCOMETgroup,inparticular,HeatherBagley,PatientandPublicInvolvementCoordinator.
WewouldalsoliketoacknowledgethehelpandadviceofProfessorBridgetYoung,OliviaLloydandHelenHansonandtheClinicalStudiesGroupconsumerrepresentativesandBSPARParentGroup,particularlySharonDouglas.
WewouldliketothankyoungpeoplefromNIHRYoungPerson'sAdvisoryGroupandtheJDMYoungPerson'sGroup,fortheiradviceonpatientquestionnairesandinformationleaflets.
Weacknowledgethesupportandcollaborationofpatient/parentsupportgroups,CureJMandMyositisUK.
WewouldliketothankKatieArnold,LawrenceBrown,KathForrestandKarenBarnesfortheiradministrativesupportforthiswork.
TheUKJDMCohortandBiomarkerstudyhasbeensupportedbygenerousgrantsfromtheWellcomeTrustUK(085860),ActionMedicalResearchUK,(SP4252),TheMyositisSupportGroupUK,ArthritisResearchUK(14518),TheHenrySmithCharity.
LWsworkissupportedinpartbyGreatOrmondStreetChildren'sCharity,theGOSH/ICHNIHRfundedBiomedicalresearchCentre(BRC)andArthritisResearchUK.
TheJDMCohortstudyisadoptedontotheComprehensiveResearchNetworkthroughtheMedicinesforChildrenResearchNetwork(www.
mcrn.
org.
uk)andissupportedbytheGOSH/ICHBiomedicalResearchCentre.
AfulllistofcontributorstotheJDCBScanbefoundontheJDRGwebsitehttp://www.
juveniledermatomyositis.
org.
uk.
Authordetails1AlderHeyChildren'sNHSFoundationTrust,EatonRoad,Liverpool,UK.
2MRCNorthWestHubforTrialsMethodologyResearch,DepartmentofBiostatistics,UniversityofLiverpool,Liverpool,UK.
3Infection,Immunology,andRheumatologySectionUCLInstituteofChildHealth,UniversityCollegeLondon,London,UK.
4GreatOrmondStreetHospitalNHSFoundationTrust,London,UK.
5CentreforAdolescentRheumatologyatUniversityCollegeLondon,UniversityCollegeLondonHospital,London,UK.
6IWKHealthCentreandDalhousieUniversity,5850UniversityAvenue,Halifax,NSB3K6R8,Canada.
7UniversitàdegliStudidiGenovaandIstitutoGianninaGaslini,ViaG.
Gaslini5,16147Genoa,Italy.
8DepartmentofWomen'sandChildren'sHealth,InstituteofTranslationalMedicine,UniversityofLiverpool,Liverpool,UK.
Received:19February2015Accepted:29May2015References1.
McCannLJ,ArnoldK,PilkingtonCA,HuberAM,RavelliA,BeardL,etal.
Developingaprovisional,internationalMinimalDatasetforJuvenileDermatomyositis:foruseinclinicalpracticetoinformresearch.
Pediatr.
Rheumatol.
2014;12:31.
2.
RiderLG,GianniniEH,Harris-LoveM,JoeG,IsenbergD,PilkingtonC,etal.
Definingclinicalimprovementinadultandjuvenilemyositis.
JRheumatol.
2003;30:603–17.
3.
RiderLG,GianniniEH,BrunnerHI,RupertoN,James-NewtonL,ReedAM,etal.
Internationalconsensusonpreliminarydefinitionsofimprovementinadultandjuvenilemyositis.
ArthritisRheum.
2004;50:2281–90.
4.
InternationalMyositisAssessmentandClinicalStudiesGroup,IMACS.
http://www.
niehs.
nih.
gov/research/resources/imacs5.
RupertoN,RavelliA,PistorioA,FerrianiV,CalvoI,GanserG,etal.
TheprovisionalPaediatricRheumatologyInternationalTrialsOrganisation/AmericanCollegeofRheumatology/EuropeanLeagueAgainstRheumatismDiseaseactivitycoresetfortheevaluationofresponsetotherapyinjuveniledermatomyositis:aprospectivevalidationstudy.
ArthritisRheum.
2008;59:4–13.
6.
RupertoN,PistorioA,RavelliA,RiderLG,PilkingtonC,OliveiraS,etal.
ThePaediatricRheumatologyInternationalTrialsOrganisationprovisionalcriteriafortheevaluationofresponsetotherapyinjuveniledermatomyositis.
ArthritisCareRes(Hoboken).
2010;62:1533–41.
7.
PaediatricRheumatologyINternationalTrialsOrganisation,PRINTO.
http://www.
printo.
it/.
8.
MartinN,KrolP,SmithS,MurrayK,PilkingtonCA,DavidsonJE,etal.
Anationalregistryforjuveniledermatomyositisandotherpaediatricidiopathicinflammatorymyopathies:10years'experience;theJuvenileDermatomyositisNational(UKandIreland)CohortBiomarkerStudyandRepositoryforIdiopathicInflammatoryMyopathies.
Rheumatology(Oxford).
2011;50:137–45.
9.
CoreOutcomeMeasuresinEffectivenessTrials(COMET)Initiativedatabasehttp://www.
cometinitiative.
org.
10.
GargonE,WilliamsonPR,AltmanDG,BlazebyJM,ClarkeM.
TheCOMETInitiativedatabase:progressandactivitiesfrom2011–2013.
Trials.
2014;15:279.
11.
OMERACT(OutcomeMeasuresinRheumatology)collaboration.
http://www.
omeract.
org/.
12.
BoersM,KirwanJR,WellsG,BeatonD,GossecL,d'AgostinoMA,etal.
DevelopingCoreOutcomeMeasurementSetsforclinicaltrials:OMERACTFilter2.
0.
JClinEpidemiol.
2013;67(7):745–53.
13.
SinhaIP,SmythRL,WilliamsonPR.
UsingtheDelphitechniquetodeterminewhichoutcomestomeasureinclinicaltrials:recommendationsforthefuturebasedonasystematicreviewofexistingstudies.
PLoSMed.
2011;8(1),e1000393.
14.
JonesJ,HunterD.
Qualitativeresearch:consensusmethodsformedicalandhealthservicesresearch.
BMJ.
1995;311(7001):376–80.
15.
CantrillJA,SibbaldB,BuetowS.
TheDelphiandnominalgrouptechniquesinhealthservicesresearch.
IntJPharmPract.
1996;4(2):67–74.
16.
RiderLG,WerthVP,HuberAM,AlexandersonH,RaoAP,RupertoN,etal.
Measuresofadultandjuveniledermatomyositis,polymyositis,andinclusionbodymyositis:PhysicianandPatient/ParentGlobalActivity,ManualMuscleTesting(MMT),HealthAssessmentQuestionnaire(HAQ)/ChildhoodHealthAssessmentQuestionnaire(C-HAQ),ChildhoodMyositisAssessmentScale(CMAS),MyositisDiseaseActivityAssessmentTool(MDAAT),DiseaseActivityScore(DAS),ShortForm36(SF-36),ChildHealthQuestionnaire(CHQ),physicianglobaldamage,MyositisDamageIndex(MDI),QuantitativeMuscleTesting(QMT),MyositisFunctionalIndex-2(FI-2),MyositisActivitiesProfile(MAP),InclusionBodyMyositisFunctionalRatingScale(IBMFRS),CutaneousDermatomyositisDiseaseAreaandSeverityIndex(CDASI),CutaneousAssessmentTool(CAT),DermatomyositisSkinSeverityIndex(DSSI),Skindex,andDermatologyLifeQualityIndex(DLQI).
ArthritisCareRes(Hoboken).
2011;63Suppl11:S118–57.
17.
ChildhoodArthritisandRheumatologyResearchAlliance,CARRA.
www.
carragroup.
org.
18.
JuvenileDermatomyositisResearchGroupUKandIreland,JDRG.
http://www.
juveniledermatomyositis.
org.
uk.
19.
PaediatricRheumatologyEuropeanSociety,PReS.
http://www.
pres.
org.
uk/.
20.
DillmanDA,SmytheJD,ChristianLM.
Mailandinternetsurveys:thetailoreddesignmethod.
2nded.
Hoboken,NJ:Wiley;2007.
21.
GuyattGH,OxmanAD,KunzR,AtkinsD,BrozekJ,VistG,etal.
GRADEguidelines:2Framingthequestionanddecidingonimportantoutcomes.
JClinEpidemiol.
2011;64(4):395–400.
22.
RiderLG,KoziolD,GianniniEH,JainMS,SmithMR,Whitney-MahoneyK,etal.
Validationofmanualmuscletestingandasubsetofeightmuscles(MMT8)foradultandjuvenileidiopathicinflammatorymyopathies.
ArthritisCareRes.
2010;62(4):465–72.
23.
StringerE,BohnsackJ,BowyerSL,GriffinTA,HuberAM,LangB,etal.
Treatmentapproachestojuveniledermatomyositis(JDM)acrossNorthAmerica:TheChildhoodArthritisandRheumatologyResearchAlliance(CARRA)JDMTreatmentSurvey.
JRheumatol.
2010;37(9):1953–61.
24.
BrownVE,PilkingtonCA,FeldmanBM,DavidsonJE,onbehalfoftheNetworkforJuvenileDermatomyositis,aworkingpartyofthePaediatricRheumatologyEuropeanSociety(PReS).
Aninternationalconsensussurveyofthediagnosticcriteriaforjuveniledermatomyositis(JDM).
Rheumatology.
2006;45:990–3.
25.
BrunnerHI,MinaR,PilkingtonC,BeresfordMW,ReiffA,LevyDM,etal.
Preliminarycriteriaforglobalflaresinchildhood-onsetsystemiclupuserythematosus.
ArthritisCareRes(Hoboken).
2011;63(9):1213–23.
McCannetal.
Trials(2015)16:268Page14of1526.
RupertoN,HanrahanLM,AlarcónGS,BelmontHM,BreyRL,BrunettaP,etal.
Internationalconsensusforadefinitionofdiseaseflareinlupus.
Lupus.
2011;20(5):453–62.
27.
NIHRCRN:Children/ArthritisResearchUKPaediatricRheumatologyClinicalStudiesGroup.
http://www.
arthritisresearchuk.
org/research/our-clinical-study-groups-and-research-strategies/paediatric-rheumatology.
aspx.
28.
MyositisUK.
http://www.
myositis.
org.
uk/.
29.
CureJMFoundation.
http://www.
curejm.
org/.
30.
MatzaLS,PatrickDL,RileyAW,AlexanderJJ,RajmilL,PleilAM,etal.
Pediatricpatient-reportedoutcomeinstrumentsforresearchtosupportmedicalproductlabeling:reportoftheISPORPROgoodresearchpracticesfortheassessmentofchildrenandadolescentstaskforce.
ValueHealth.
2013;16(4):461–79.
31.
SMOG(simplifiedmeasureofgobbledygook)grading.
http://www.
niace.
org.
uk/misc/SMOG-calculator/smogcalc.
php.
32.
TheBritishSocietyforPaediatricandAdolescentRheumatology(BSPAR).
http://www.
bspar.
org.
uk/.
33.
NationalInstituteforHealthResearch(NIHR)ClinicalResearchNetwork(CRN)YoungPerson'sAdvisoryGroup.
http://www.
crn.
nihr.
ac.
uk/children/pcpie/young-persons-advisory-group/.
34.
RupertoN,RavelliA,MurrayKJ,LovellDJ,Andersson-GareB,FeldmanBM,etal.
Preliminarycoresetsofmeasuresfordiseaseactivityanddamageassessmentinjuvenilesystemiclupuserythematosusandjuveniledermatomyositis.
Rheumatology.
2003;42:1452–9.
35.
NugentJ,RupertoN,GraingerJ,MachadoC,SawhneyS,BaildamE,etal.
TheBritishversionoftheChildhoodHealthAssessmentQuestionnaire(CHAQ)andtheChildHealthQuestionnaire(CHQ).
ClinExpRheumatol.
2001;2001(19):S163–7.
36.
VarnierGC,FerrariC,ConsolaroA,MarafonD,PilkingtonC,MaillardS,etal.
Introducinganewapproachtothecareofjuveniledermatomyositis:thejuveniledermatomyositismultidimensionalassessmentreport.
PediatrRheumato.
2013;11Suppl2:25.
37.
WulffraatNM,VastertB,theSHAREconsortium.
Timetoshare.
PediatrRheumatol.
2013;11(5)http://www.
ped-rheum.
com/content/11/1/5.
38.
MurphyMK,BlackNA,LampingDL,McKeeCM,SandersonCF,AskhamJ,MarteauT.
Consensusdevelopmentmethods,andtheiruseinclinicalguidelinedevelopment:areview.
HealthTechnologyAssessment.
1998.
2(3).
39.
Euromyositis.
http://Euromyositis.
eu40.
LundbergIE,SvenssonJ.
Registriesinidiopathicinflammatorymyopathies.
CurrOpinRheumatol.
2013;25:729–34.
41.
PatrickDL,BurkeLB,GwaltneyCJ,LeidyNK,MartinML,MolsenE,etal.
Contentvalidity–establishingandreportingtheevidenceinnewlydevelopedPatient-ReportedOutcomes(PRO)instrumentsformedicalproductevaluation:ISPORPROGoodResearchPracticesTaskForceReport:Part1–ElicitingconceptsforanewPROinstrument.
ValueHealth.
2011;14:967–77.
SubmityournextmanuscripttoBioMedCentralandtakefulladvantageof:ConvenientonlinesubmissionThoroughpeerreviewNospaceconstraintsorcolorgurechargesImmediatepublicationonacceptanceInclusioninPubMed,CAS,ScopusandGoogleScholarResearchwhichisfreelyavailableforredistributionSubmityourmanuscriptatwww.
biomedcentral.
com/submitMcCannetal.
Trials(2015)16:268Page15of15
- sciencepcpie相关文档
- volumepcpie
- Brsenfhigkeitpcpie
- grapcpie
- Todaypcpie
- 自己的达贝妮:pcpie总裁
- pcpie80后的那两个很有钱的美女叫什么?
云雀云(larkyun)低至368元/月,广州移动1Gbps带宽VDS(带100G防御),常州联通1Gbps带宽VDS
云雀云(larkyun)当前主要运作国内线路的机器,最大提供1Gbps服务器,有云服务器(VDS)、也有独立服务器,对接国内、国外的效果都是相当靠谱的。此外,还有台湾hinet线路的动态云服务器和静态云服务器。当前,larkyun对广州移动二期正在搞优惠促销!官方网站:https://larkyun.top付款方式:支付宝、微信、USDT广移二期开售8折折扣码:56NZVE0YZN (试用于常州联...
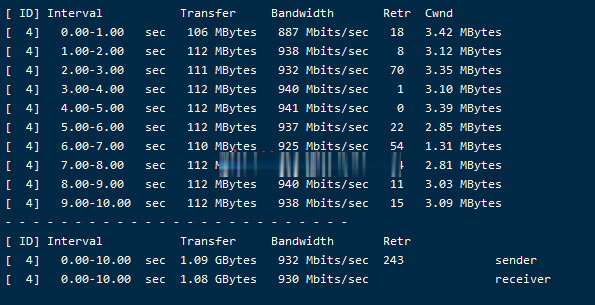
老薛主机入门建站月付34/月,年付345元,半价香港VPS主机
老薛主机怎么样?老薛主机这个商家有存在有一些年头。如果没有记错的话,早年老薛主机是做虚拟主机业务的,还算不错在异常激烈的市场中生存到现在,应该算是在众多商家中早期积累到一定的用户群的,主打小众个人网站业务所以能持续到现在。这不,站长看到商家有在进行夏季促销,比如我们很多网友可能有需要的香港vps主机季度及以上可以半价优惠,如果有在选择不同主机商的香港机房的可以看看老薛主机商家的香港vps。点击进入...
GigsGigsCloud:$16/月KVM-1GB/30GB/1TB/1.6T高防/洛杉矶CN2 GIA+AS9929
GigsGigsCloud是一家成立于2015年老牌国外主机商,提供VPS主机和独立服务器租用,数据中心包括美国洛杉矶、中国香港、新加坡、马来西亚和日本等。商家VPS主机基于KVM架构,绝大部分系列产品中国访问速度不错,比如洛杉矶机房有CN2 GIA、AS9929及高防线路等。目前Los Angeles - SimpleCloud with Premium China DDOS Protectio...
pcpie为你推荐
-
软银收购wework校内网被软银收购后会泄露我国几千万大学生的资料给日本吗???视频制作软件哪个好免费的视频剪辑软件用哪个好?免费阅读小说app哪个好哪个手机小说app比较好用呢?机械表和石英表哪个好自动石英表与全自动机械表哪个好dnf魔枪士转职哪个好dnf魔枪士转职哪个适合平民玩红茶和绿茶哪个好红茶好还是绿茶好?美国国际东西方大学美国大学一年学费是多少?空间登录器用什么登录器可以登录QQ(除了QQ登录器)考生个人空间登录湖南自考所有成绩查询怎么查dns服务器未响应DNS服务器未响应