higherPeriodic Trends Worksheet - Faculty Access for the Web
Na me_________________________________________________________________________________Date_
Periodic Trends Worksheet
Directions:Use your notes to answer thefollowing questions.
1.What is atomic radius?
2. Rank the following elements by increasing atomic radius: carbon,aluminum,oxygen,potassium.
3. Define Ionization energy.
4.Why is the second ionization energy always larger than the first?
5.Why does fluorine have higher ionization energy than iodine?
6.What are valence electrons?
7.Why do elements in the same family generally have similar properties?
8. Indicate whether the following properties increase or decrease from left to right across the periodic table.a. atomic radius (exc luding nob le gases)b. first ionization energy
1
Na me_________________________________________________________________________________Date_
9.What trend in atomic radius occurs down a group on the periodic table?What causes this trend?
10.What trend in ionization energy occurs across a period on the periodic table?What causes this trend?
11.Circle the atom in each pair that has the largest atomic radius.a. Al or Bb. Na or Alc. S or Od. O or Fe. Br or Clf. Mg or Ca
12. Circle the atom in each pair that has the greater ionization energy.a. Li or Beb. Ca or Bac. Na or Kd. P or Are. Cl or Sif. Li or K
13.Which ofthe following in each pair has the larger atomic radius?
1. LiorK
________
2. Ca orNi
________
3. Ga or B
________
4. O or C
________
5. Be or Ba
________
6. SiorS
________
7. Fe or Kr
________
14.Which ofthe following in each pair has the larger ionization energy?
1. LiorK
________
2. Ca orNi
________
3. Ga or B
________
4. O or C
________
5. Be or Ba
________
6. SiorS
7. Fe or Kr
________
2
Na me_________________________________________________________________________________Date_
15.Which ion will have the smaller atomic radius?
1. K+or O2-
2. Ba2+or I-________
3. Al3+or Cl-________
4. K+ or Ca+2 ___
5. P-3 or S2-_____________
16.Why is the last answer S2-?Explain
3
- higherPeriodic Trends Worksheet - Faculty Access for the Web相关文档
- periodPERIODIC TABLE TRENDS - SCHOOLinSITES Web hosting …元素周期表的趋势schoolinsites网页寄存…
- PCTechnological Trends of Distance Learning From Web-Based to WAP-Based
- 网页Mobile Web Design Trends and Best Practices
- WebWandererHow Is My Web Community Developing Monitoring Trends In Web…
- specificInventory- likely to be underestimated three interesting web design trends
- demonstrateGrowing Trends article - NYSTA Web Site
CloudCone月付$48,MC机房可小时付费
CloudCone商家在前面的文章中也有多次介绍,他们家的VPS主机还是蛮有特点的,和我们熟悉的DO、Linode、VuLTR商家很相似可以采用小时时间计费,如果我们不满意且不需要可以删除机器,这样就不扣费,如果希望用的时候再开通。唯独比较吐槽的就是他们家的产品太过于单一,一来是只有云服务器,而且是机房就唯一的MC机房。CloudCone 这次四周年促销活动期间,商家有新增独立服务器业务。同样的C...

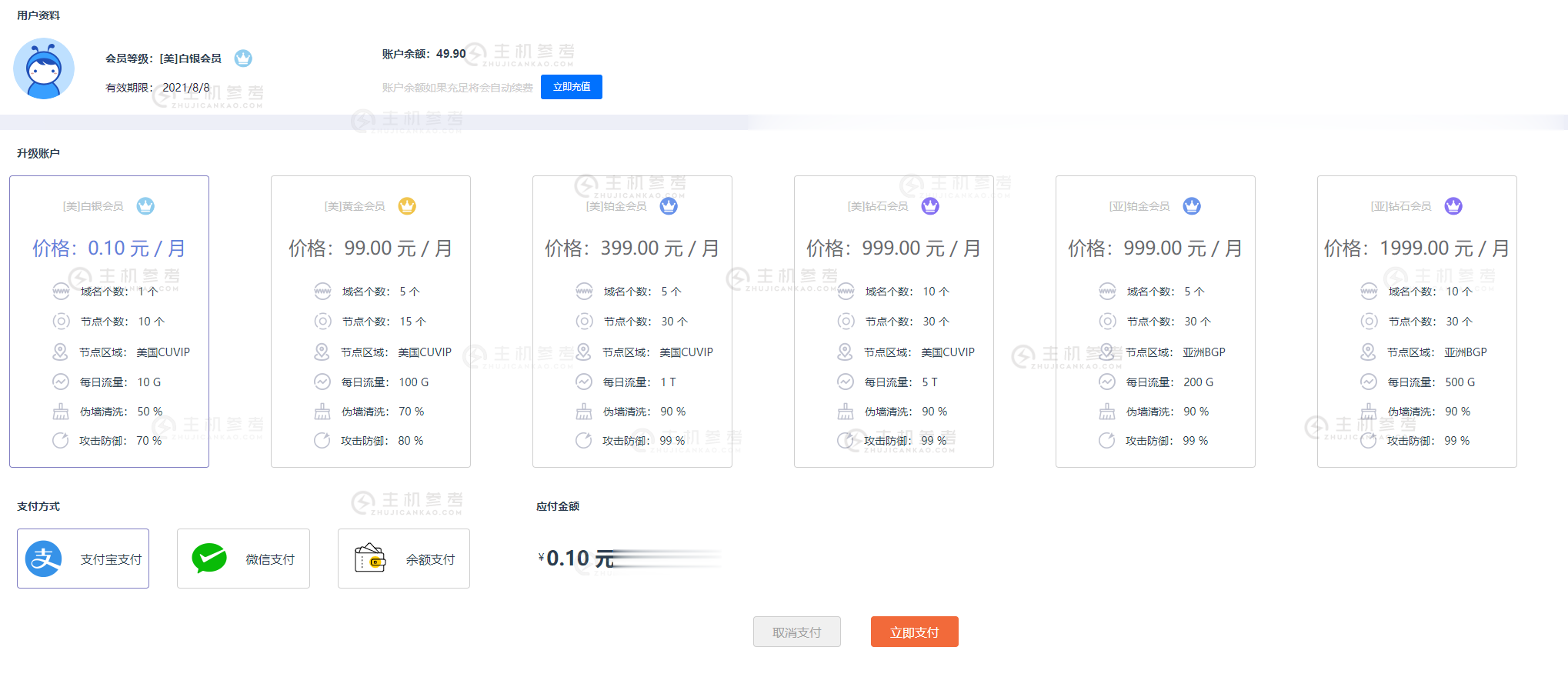
FBICDN,0.1元解决伪墙/假墙攻击,超500 Gbps DDos 防御,每天免费流量高达100G,免费高防网站加速服务
最近很多网站都遭受到了伪墙/假墙攻击,导致网站流量大跌,间歇性打不开网站。这是一种新型的攻击方式,攻击者利用GWF规则漏洞,使用国内服务器绑定host的方式来触发GWF的自动过滤机制,造成GWF暂时性屏蔽你的网站和服务器IP(大概15分钟左右),使你的网站在国内无法打开,如果攻击请求不断,那么你的网站就会是一个一直无法正常访问的状态。常规解决办法:1,快速备案后使用国内服务器,2,使用国内免备案服...
香港站群多ip服务器多少钱?零途云香港站群云服务器怎么样?
香港站群多ip服务器多少钱?想做好站群的SEO优化,最好给每个网站都分配一个独立IP,这样每个网站之间才不会受到影响。对做站群的站长来说,租用一家性价比高且提供多IP的香港多ip站群服务器很有必要。零途云推出的香港多ip站群云服务器多达256个IP,可以满足站群的优化需求,而且性价比非常高。那么,香港多ip站群云服务器价格多少钱一个月?选择什么样的香港多IP站群云服务器比较好呢?今天,小编带大家一...

-
免费卡巴斯基杀毒软件除了卡巴斯基,还有哪些杀毒软件游戏加速器哪个好网游加速器那个好?录音软件哪个好录音软件哪个好录音软件哪个好录音软件哪个好用又简单核芯显卡与独立显卡哪个好核芯显卡和独立显卡有什么区别?最好的是哪个?网校哪个好哪个网校比较好?美国国际集团深圳500强企业都有哪些?网通dns服务器地址湖北省鄂州市葛店镇DNS服务器IP地址是多少强生美瞳月抛强生美瞳有月抛的吗广东联通彩铃广东联通卡用短信怎样开通彩铃?