www.sciencemag.org/cgi/content/full/316/5821/120/DC1
akiba-online 时间:2021-03-20 阅读:()
SupportingOnlineMaterialforAnATPGateControlsTubulinBindingbytheTetheredHeadofKinesin-1MariaC.
Alonso,DouglasR.
Drummond,SusanKain,JuliaHoeng,LindaAmos,RobertA.
Cross1**Towhomcorrespondenceshouldbeaddressed.
E-mail:r.
cross@mcri.
ac.
ukPublished6April2007,Science316,120(2007)DOI:10.
1126/science.
1136985ThisPDFfileincludes:MaterialsandMethodsSOMTextFigs.
S1toS4ReferencesPageS1SSupplementaryMaterial|Alonsoetal|ATP-GatingmechanismofKinesinSupplementaryMethods1.
Proteins&proteinchemicalmethods1.
1Proteins|Nkin460GSTcontainingtheN-terminal460aminoacidsofNeurosporakinesin-1andaC-terminalGSTfusionwaspreparedasdescribed(1).
Rat430GSTprotein(2)usedinthesteadystateATPaseassayscontainedtheequivalent430N-terminalaminoacidsofratKinesin-1andaC-terminalGSTfusion.
ThisproteinwasagiftfromDrIsabelleCrevel.
RatrK430asusedintheFPLCgelfiltrationandthekineticsexperimentswasuntagged.
TheinsertforthisconstructwasPCRamplifiedfromafulllengthratkinesin(kif5cclone)originallygiventousbyScottBrady.
StartingOligo:5'-CCGCTCTACATATGGCGGACCCAGCCGAATGCAGC-3'EndingOligo:5'-CCGACTAGTCTAGAATTCCTTGTCATCCAGCTG-3'.
ThePCRproductwasdigestedwithNdeI/SpeIandligatedintopET17b.
Expressionandpurificationoftheproteinwasaccordingto(3).
PigbraintubulinwaspreparedaccordingtoMitchisonandKirschner(4)(http://mitchison.
med.
harvard.
edu/protocols.
htm)andS.
pombetubulinbyamodificationofthemethodofDavisetal(5)tobedescribedindetailelsewhere.
PurifiedtubulinsweredesaltedintoPEMcontaining20μMGDPbeforestorageinliquidnitrogen.
ProteinconcentrationsweredeterminedbyUVabsorptionscanofsamplesofproteindissolvedin6Mguanidinehydrochloride,correctingforbackgroundscatteringbysubtractingascanofbufferalone,andassumingfullnucleotideoccupancy.
1.
2SteadystateATPase|ThemicrotubuleortubulinheterodimerstimulatedATPaseactivityofkinesinwasmeasuredat25Cusingapyruvatekinase/lactatedehydrogenaselinkedassayasdescribed(6),exceptthatmicrotubuleswereassembledinPEM(7)containing1mMGMPCPP,pelletedandresuspendedinPEMcontaining10nMGMPCPPbeforedilutionintothelinkedassaybuffer(20mMPIPES,pH6.
9,5mMMgCl2,1mMDTT,0.
1mgml-1BSA).
TubulinheterodimersweredilutedfromastockinPEMcontaining20μMGDP.
NAD/HabsorptionmeasurementsweremadeinaCary50spectrophotometerusingdisposable50μlUVtransparentcuvettes(Eppendorf).
ValuesforVmaxandKmwereobtainedbyleastsquaresfittingtoplotsofATPaseversustubulinheterodimerconcentration,usingKaleidagraph3.
6.
4(SynergySoftware).
ATPasevaluesareperkinesinhead.
1.
3Superose12gelfiltration|Stockkinesin(typically69Mheadsconcentrationin20%glycerol,20mMphosphatebufferpH7.
0,2mMMgCl2,0.
3MNaCl,0.
1mMDTT)wasmixedwithstocktubulin(typically58μMheterodimersin50mMPIPESpH6.
9,0.
2mMMgCl2,1mMEGTA,0.
1μMNa-GTP,30%glycerol)andadjustedtoafinalvolumeof240landfinalconcentrationsof6.
5μMkinesinheadsand13μMtubulinheterodimers,witheithernoaddednucleotide,or2mMAMPPNPor2mMADPbyadding10xcolumnbufferandwaterasnecessary.
Themixturewasincubatedfor10minsoniceandcentrifugedbrieflyinamicrofuge(thiswasprecautionary:nopelletwasobtained).
Theentire240μlsamplewasthenappliedtoaSuperoseHR10/300GLcolumnheldat4Candrunningat0.
5mlmin-1.
Thecolumnbufferwas50mMPIPESpH6.
9,2mMMgCl2,1mMEGTA;pluseithernothingor2mMADPor0.
2mMAMPPNP.
0.
3mlfractionswerecollected.
ThereducedconcentrationofAMPPNPinthecolumnbufferwasfoundtobeadequateandwasusedforreasonsofcost.
Elutionpatternsweremonitoredat290nmtoavoidthelargenucleotidecontributiontothe280nmabsorbance.
ThekinesinabsorbanceislowbecausetherearefewtryptophansintherK430sequence.
Thisisconvenientbecauseitmeansthatthetubulinbehaviourdominatestheelutionprofilesfromthemixtures.
PageS2S1.
4Gelelectrophoresisanddensitometry|SamplesfromthepeakfractionswererunonInvitrogenNuPAGE10%bis-trisprecast15wellgelsandruninMOPS(Invitrogen)bufferat200Vfor1.
5hours.
GelswerestainedwithSimplyBlue(Invitrogen)andimagedwithagreyscaleCCDcamera.
QuantitationwasinitiallydoneusingImageJ(Rasband,W.
S.
(1997-2006);http://rsb.
info.
nih.
gov/ij).
Integratedopticaldensityvaluesforeachbandwerecorrectedbysubtractingtheintegratedopticaldensityofacorrespondinglocalareaoffeaturelessgel.
Thesystemwascalibratedusinganopticaldensitywedge.
Inlaterwork,weusedthesyproorgangefluorescentstainingsystem(MolecularProbes,methodexactlyaspermanufacturer'sinstructions)incombinationwithaPhosphorimager(MolecuarDynamics)andBioRadImageQuantsoftware.
Thetwoapproachesgavecomparableresults.
ThenumbersquotedinthetextreferonlytothePhosphorimager/Syproreddata.
1.
5MantATPturnoverexperiments|Stocktubulin(seeabove)wasdialysedfor30minutesagainstalargeexcessvolumeof50mMPIPESpH6.
9,0.
2mMMgCl2,1mMEGTA,10%glyceroltoremoveunboundguanidinenucleotides.
TheconcentrationofthedialysedproteinwasdeterminedbyUVabsorptionscan(seeabove).
Enzymereactionsweredonein20mMPIPESpH6.
9,2mMMgCl2,usingquartzfluorimetercuvettes.
FluorescencetransientswererecordedwithaCaryEclipsefluorimeter.
Atthestartofthereaction,cuvettescontained1μMMantATPin20mMPIPESpH6.
9,2mMMgCl2.
Tothisweaddedkinesintoafinalconcentrationof1μMkinesinheads,andinsomeexperimentstubulintoafinalconcentrationof2μMtubulinheterodimers.
Thenwe"chased"byaddingeither100mMNa-ATPor100mMAMPPNP,to1mMfinalconcentration.
Thefinalvolumeineverycasewas600μl.
Experimentsinwhich4Moftubulinwasaddedyieldedessentiallyidenticalresults(notshown).
PageS3S2.
SupplementaryData.
2.
1Superose12gelfiltrationofkinesin-tubulinmixturesFigureS1|Superose12chromatographyofkinesin-tubulinmixturesThedatawereobtainedasdescribedaboveandforFig.
2inthemaintext,andtheaxesareequivalent.
Thedifferenceinthiscaseisthatamixingratioof1tubulinheterodimerperkinesinheadwasused.
Thetubulinpeak(therightmostpeakvisibleinFig.
S1AandFig.
S1B)isthenentirelyshiftedtoanearlierelutionpositioninFig.
S1C.
PageS4S2.
2Dockingtubulinheterodimerstotheratkinesindimer(3KIN)crystalstructureFigureS2(below)showsthatthetubulinbindingsitesof3KINarepositionedsothatweretubulinheterodimerstobindtothisstructure,theywouldbespacedwellapartandwouldnotclashwithoneanother.
In3KIN,bothheadsareoccupiedbyADP,andbothnecklinkersaredocked.
OurdatashowthatonlyonetubulinheterodimercanbindtoakinesindimerintheabsenceofAMPPNP.
FittingofcryoEMdatasuggeststhepossibilitythatthetubulinbindingsiteinthesecondheadisphysicallymasked(below).
Furtherworkwillberequiredtodeterminethestructuralmechanismbywhichthetubulinbindingsiteonthesecondheadisblocked.
FigureS2|Dockingtubulinheterodimerstotheratkinesindimer(3KIN)crystalstructurePageS5S2.
3Dockingakinesincrystalstructureintotheparkedheadofacryo-EMstructureThemicrotubuleboundheadThecryo-EMsurfacecontourmapshowninFig.
S3isof15-pfMTsdecoratedwithdimericratkinesin-1intheabsenceoffreenucleotide(8),expectedtocorrespondtotheATP-waitingdwellstateinthewalkingmechanism.
Thismaphasaresolutionof~30.
SupplementaryFigs.
S4A-Cshowdockingofamodelcomprisingatubulinprotofilamentwithonekinesinheadbound(whitepolypeptidebackbone)intotheCryoEMmap(bluenet).
ThismodelcomplexcomprisestubulinandthemotordomainofKar3inanorientationderivedinseparateexperimentsbydockingintoahighresolutioncryo-EMmap(9).
Themaincontactwithtubulinismadebyhelixα4ofthemotordomain,aswasalsofoundforKif1A(10).
Thecrystalstructuresofkinesin-1'scanbealignedtothoseofKar3withr.
m.
s.
differencesof1.
5-3,sothetubulin-Kar3complexservesasasufficientmodelhere.
ThetetheredheadWethenconsideredthepositionofthesecond(tethered)head.
Fittingwasdonebyeyeusingoneheadoftheratkinesindimer3KIN,requiringthatthetwostrandsofthecoiledcoilneckofthedimerremainclose.
Figs.
S4A-Cshowthetetheredheadingreen.
Thisapproachyielded2possibledockingsforthesecondhead,AandB.
Boththesedockingsforthesecondmotordomainhavetheα-helicalneckofthesecondmotordomaininroughlytherightregiontopairintoacoiledcoilwiththatofthefirstmotordomain.
Fig.
S4Ashowsthefrontviewofthesetwoalternativefits.
ItisclearthatdockingAprovidesthebetterfit.
Yellowarrowspointtothestretchofhelixthatwouldformhalfofthecoiled-coilstalk.
Thedifferencesbetweenthedockingsarelessdistinctinotherviews(Fig.
S4B&S4C)sincetheoverallshapeofthemotordomainisroughlytriangular,withupto6possibledockings.
CaveatIndoingthisfitting,weconsideredthepossibilitythatthebulkofthekinesin-1motordomainmaymoverelativetoα4,ashasbeenobservedforKif1A(10,11),butnotforKar3(9).
Ifsuchamovementoccurred,itwouldnoticeablyaffectthepositionofthealphahelicalcoiledcoilstalkbutnotthespaceavailablefordockingthe2ndhead.
TheworkonKar3suggeststhatthetopoftheboundmotordomainundergoesasignificantconformationalchangeintheapostatebutthereisnocrystalstructureforthisstate.
Accordingly,thepossibilitythatthemainpartoftheheadmightshiftrelativetoalpha4limitstheprecisionofthefitting.
Specifically,thereisuncertaintyabouttheprecisepositionoftheendofthenecklinkerofthethemicrotubule-boundhead.
Nonethelesstheapproximatefitobtainedisrobust,becausetheelongatedshapeoftheheadincombinationwitharequirementforcloseappositionofthetwonecksprovidesasufficientconstraint.
PageS6SConclusionsHigherresolutionstudiesoftubulinanddimerickinesinarerequiredtoestablishthepreciseandunequivocalorientationforthesecondhead.
Itisnonethelessclearthatthemostreasonablefit(dockingA),leadstopartialocclusionofthemicrotubulebindingsurfaceofthetetheredhead,suggestingthattubulinbindingbythetetheredheadisinhibitedinthedwellstatebyparkingthetetheredheadagainsttheboundheadsoastomaskitstubulinbindingsite.
AlonsoetalSupplementaryFig.
S3.
CryoEMmapofthedwellstate(8)PageS7SAlonsoetalSupplementaryFig.
S4.
FittingthecryoEMmap.
Fig.
S4A|FrontViewDockingADockingBPageS8SFig.
S4B|SideViewDockingADockingBPageS9SFig.
S4C|TopViewDockingADockingBSupplementaryReferences1.
I.
Crevel,N.
Carter,M.
Schliwa,R.
Cross,EmboJ18,5863(1999).
2.
I.
M.
Crevel,A.
Lockhart,R.
A.
Cross,JMolBiol273,160(1997).
3.
A.
Lockhart,I.
M.
Crevel,R.
A.
Cross,JMolBiol249,763(1995).
4.
T.
Mitchison,M.
Kirschner,Nature312,232(Nov15-21,1984).
5.
A.
Davis,C.
R.
Sage,L.
Wilson,K.
W.
Farrell,Biochemistry32,8823(Aug31,1993).
6.
A.
Lockhart,R.
A.
Cross,EmboJ13,751(1994).
7.
J.
W.
Walker,G.
P.
Reid,J.
A.
McCray,D.
R.
Trentham,JAmChemSoc110,7170(1988).
8.
K.
Hirose,J.
Lowe,M.
Alonso,R.
A.
Cross,L.
A.
Amos,MolBiolCell10,2063(1999).
9.
K.
Hirose,E.
Akimaru,T.
Akiba,S.
A.
Endow,L.
A.
Amos,MolCell23,913(Sep15,2006).
10.
R.
Nitta,M.
Kikkawa,Y.
Okada,N.
Hirokawa,Science305,678(Jul30,2004).
11.
M.
Kikkawa,N.
Hirokawa,EmboJ(Aug31,2006).
tubulin
Alonso,DouglasR.
Drummond,SusanKain,JuliaHoeng,LindaAmos,RobertA.
Cross1**Towhomcorrespondenceshouldbeaddressed.
E-mail:r.
cross@mcri.
ac.
ukPublished6April2007,Science316,120(2007)DOI:10.
1126/science.
1136985ThisPDFfileincludes:MaterialsandMethodsSOMTextFigs.
S1toS4ReferencesPageS1SSupplementaryMaterial|Alonsoetal|ATP-GatingmechanismofKinesinSupplementaryMethods1.
Proteins&proteinchemicalmethods1.
1Proteins|Nkin460GSTcontainingtheN-terminal460aminoacidsofNeurosporakinesin-1andaC-terminalGSTfusionwaspreparedasdescribed(1).
Rat430GSTprotein(2)usedinthesteadystateATPaseassayscontainedtheequivalent430N-terminalaminoacidsofratKinesin-1andaC-terminalGSTfusion.
ThisproteinwasagiftfromDrIsabelleCrevel.
RatrK430asusedintheFPLCgelfiltrationandthekineticsexperimentswasuntagged.
TheinsertforthisconstructwasPCRamplifiedfromafulllengthratkinesin(kif5cclone)originallygiventousbyScottBrady.
StartingOligo:5'-CCGCTCTACATATGGCGGACCCAGCCGAATGCAGC-3'EndingOligo:5'-CCGACTAGTCTAGAATTCCTTGTCATCCAGCTG-3'.
ThePCRproductwasdigestedwithNdeI/SpeIandligatedintopET17b.
Expressionandpurificationoftheproteinwasaccordingto(3).
PigbraintubulinwaspreparedaccordingtoMitchisonandKirschner(4)(http://mitchison.
med.
harvard.
edu/protocols.
htm)andS.
pombetubulinbyamodificationofthemethodofDavisetal(5)tobedescribedindetailelsewhere.
PurifiedtubulinsweredesaltedintoPEMcontaining20μMGDPbeforestorageinliquidnitrogen.
ProteinconcentrationsweredeterminedbyUVabsorptionscanofsamplesofproteindissolvedin6Mguanidinehydrochloride,correctingforbackgroundscatteringbysubtractingascanofbufferalone,andassumingfullnucleotideoccupancy.
1.
2SteadystateATPase|ThemicrotubuleortubulinheterodimerstimulatedATPaseactivityofkinesinwasmeasuredat25Cusingapyruvatekinase/lactatedehydrogenaselinkedassayasdescribed(6),exceptthatmicrotubuleswereassembledinPEM(7)containing1mMGMPCPP,pelletedandresuspendedinPEMcontaining10nMGMPCPPbeforedilutionintothelinkedassaybuffer(20mMPIPES,pH6.
9,5mMMgCl2,1mMDTT,0.
1mgml-1BSA).
TubulinheterodimersweredilutedfromastockinPEMcontaining20μMGDP.
NAD/HabsorptionmeasurementsweremadeinaCary50spectrophotometerusingdisposable50μlUVtransparentcuvettes(Eppendorf).
ValuesforVmaxandKmwereobtainedbyleastsquaresfittingtoplotsofATPaseversustubulinheterodimerconcentration,usingKaleidagraph3.
6.
4(SynergySoftware).
ATPasevaluesareperkinesinhead.
1.
3Superose12gelfiltration|Stockkinesin(typically69Mheadsconcentrationin20%glycerol,20mMphosphatebufferpH7.
0,2mMMgCl2,0.
3MNaCl,0.
1mMDTT)wasmixedwithstocktubulin(typically58μMheterodimersin50mMPIPESpH6.
9,0.
2mMMgCl2,1mMEGTA,0.
1μMNa-GTP,30%glycerol)andadjustedtoafinalvolumeof240landfinalconcentrationsof6.
5μMkinesinheadsand13μMtubulinheterodimers,witheithernoaddednucleotide,or2mMAMPPNPor2mMADPbyadding10xcolumnbufferandwaterasnecessary.
Themixturewasincubatedfor10minsoniceandcentrifugedbrieflyinamicrofuge(thiswasprecautionary:nopelletwasobtained).
Theentire240μlsamplewasthenappliedtoaSuperoseHR10/300GLcolumnheldat4Candrunningat0.
5mlmin-1.
Thecolumnbufferwas50mMPIPESpH6.
9,2mMMgCl2,1mMEGTA;pluseithernothingor2mMADPor0.
2mMAMPPNP.
0.
3mlfractionswerecollected.
ThereducedconcentrationofAMPPNPinthecolumnbufferwasfoundtobeadequateandwasusedforreasonsofcost.
Elutionpatternsweremonitoredat290nmtoavoidthelargenucleotidecontributiontothe280nmabsorbance.
ThekinesinabsorbanceislowbecausetherearefewtryptophansintherK430sequence.
Thisisconvenientbecauseitmeansthatthetubulinbehaviourdominatestheelutionprofilesfromthemixtures.
PageS2S1.
4Gelelectrophoresisanddensitometry|SamplesfromthepeakfractionswererunonInvitrogenNuPAGE10%bis-trisprecast15wellgelsandruninMOPS(Invitrogen)bufferat200Vfor1.
5hours.
GelswerestainedwithSimplyBlue(Invitrogen)andimagedwithagreyscaleCCDcamera.
QuantitationwasinitiallydoneusingImageJ(Rasband,W.
S.
(1997-2006);http://rsb.
info.
nih.
gov/ij).
Integratedopticaldensityvaluesforeachbandwerecorrectedbysubtractingtheintegratedopticaldensityofacorrespondinglocalareaoffeaturelessgel.
Thesystemwascalibratedusinganopticaldensitywedge.
Inlaterwork,weusedthesyproorgangefluorescentstainingsystem(MolecularProbes,methodexactlyaspermanufacturer'sinstructions)incombinationwithaPhosphorimager(MolecuarDynamics)andBioRadImageQuantsoftware.
Thetwoapproachesgavecomparableresults.
ThenumbersquotedinthetextreferonlytothePhosphorimager/Syproreddata.
1.
5MantATPturnoverexperiments|Stocktubulin(seeabove)wasdialysedfor30minutesagainstalargeexcessvolumeof50mMPIPESpH6.
9,0.
2mMMgCl2,1mMEGTA,10%glyceroltoremoveunboundguanidinenucleotides.
TheconcentrationofthedialysedproteinwasdeterminedbyUVabsorptionscan(seeabove).
Enzymereactionsweredonein20mMPIPESpH6.
9,2mMMgCl2,usingquartzfluorimetercuvettes.
FluorescencetransientswererecordedwithaCaryEclipsefluorimeter.
Atthestartofthereaction,cuvettescontained1μMMantATPin20mMPIPESpH6.
9,2mMMgCl2.
Tothisweaddedkinesintoafinalconcentrationof1μMkinesinheads,andinsomeexperimentstubulintoafinalconcentrationof2μMtubulinheterodimers.
Thenwe"chased"byaddingeither100mMNa-ATPor100mMAMPPNP,to1mMfinalconcentration.
Thefinalvolumeineverycasewas600μl.
Experimentsinwhich4Moftubulinwasaddedyieldedessentiallyidenticalresults(notshown).
PageS3S2.
SupplementaryData.
2.
1Superose12gelfiltrationofkinesin-tubulinmixturesFigureS1|Superose12chromatographyofkinesin-tubulinmixturesThedatawereobtainedasdescribedaboveandforFig.
2inthemaintext,andtheaxesareequivalent.
Thedifferenceinthiscaseisthatamixingratioof1tubulinheterodimerperkinesinheadwasused.
Thetubulinpeak(therightmostpeakvisibleinFig.
S1AandFig.
S1B)isthenentirelyshiftedtoanearlierelutionpositioninFig.
S1C.
PageS4S2.
2Dockingtubulinheterodimerstotheratkinesindimer(3KIN)crystalstructureFigureS2(below)showsthatthetubulinbindingsitesof3KINarepositionedsothatweretubulinheterodimerstobindtothisstructure,theywouldbespacedwellapartandwouldnotclashwithoneanother.
In3KIN,bothheadsareoccupiedbyADP,andbothnecklinkersaredocked.
OurdatashowthatonlyonetubulinheterodimercanbindtoakinesindimerintheabsenceofAMPPNP.
FittingofcryoEMdatasuggeststhepossibilitythatthetubulinbindingsiteinthesecondheadisphysicallymasked(below).
Furtherworkwillberequiredtodeterminethestructuralmechanismbywhichthetubulinbindingsiteonthesecondheadisblocked.
FigureS2|Dockingtubulinheterodimerstotheratkinesindimer(3KIN)crystalstructurePageS5S2.
3Dockingakinesincrystalstructureintotheparkedheadofacryo-EMstructureThemicrotubuleboundheadThecryo-EMsurfacecontourmapshowninFig.
S3isof15-pfMTsdecoratedwithdimericratkinesin-1intheabsenceoffreenucleotide(8),expectedtocorrespondtotheATP-waitingdwellstateinthewalkingmechanism.
Thismaphasaresolutionof~30.
SupplementaryFigs.
S4A-Cshowdockingofamodelcomprisingatubulinprotofilamentwithonekinesinheadbound(whitepolypeptidebackbone)intotheCryoEMmap(bluenet).
ThismodelcomplexcomprisestubulinandthemotordomainofKar3inanorientationderivedinseparateexperimentsbydockingintoahighresolutioncryo-EMmap(9).
Themaincontactwithtubulinismadebyhelixα4ofthemotordomain,aswasalsofoundforKif1A(10).
Thecrystalstructuresofkinesin-1'scanbealignedtothoseofKar3withr.
m.
s.
differencesof1.
5-3,sothetubulin-Kar3complexservesasasufficientmodelhere.
ThetetheredheadWethenconsideredthepositionofthesecond(tethered)head.
Fittingwasdonebyeyeusingoneheadoftheratkinesindimer3KIN,requiringthatthetwostrandsofthecoiledcoilneckofthedimerremainclose.
Figs.
S4A-Cshowthetetheredheadingreen.
Thisapproachyielded2possibledockingsforthesecondhead,AandB.
Boththesedockingsforthesecondmotordomainhavetheα-helicalneckofthesecondmotordomaininroughlytherightregiontopairintoacoiledcoilwiththatofthefirstmotordomain.
Fig.
S4Ashowsthefrontviewofthesetwoalternativefits.
ItisclearthatdockingAprovidesthebetterfit.
Yellowarrowspointtothestretchofhelixthatwouldformhalfofthecoiled-coilstalk.
Thedifferencesbetweenthedockingsarelessdistinctinotherviews(Fig.
S4B&S4C)sincetheoverallshapeofthemotordomainisroughlytriangular,withupto6possibledockings.
CaveatIndoingthisfitting,weconsideredthepossibilitythatthebulkofthekinesin-1motordomainmaymoverelativetoα4,ashasbeenobservedforKif1A(10,11),butnotforKar3(9).
Ifsuchamovementoccurred,itwouldnoticeablyaffectthepositionofthealphahelicalcoiledcoilstalkbutnotthespaceavailablefordockingthe2ndhead.
TheworkonKar3suggeststhatthetopoftheboundmotordomainundergoesasignificantconformationalchangeintheapostatebutthereisnocrystalstructureforthisstate.
Accordingly,thepossibilitythatthemainpartoftheheadmightshiftrelativetoalpha4limitstheprecisionofthefitting.
Specifically,thereisuncertaintyabouttheprecisepositionoftheendofthenecklinkerofthethemicrotubule-boundhead.
Nonethelesstheapproximatefitobtainedisrobust,becausetheelongatedshapeoftheheadincombinationwitharequirementforcloseappositionofthetwonecksprovidesasufficientconstraint.
PageS6SConclusionsHigherresolutionstudiesoftubulinanddimerickinesinarerequiredtoestablishthepreciseandunequivocalorientationforthesecondhead.
Itisnonethelessclearthatthemostreasonablefit(dockingA),leadstopartialocclusionofthemicrotubulebindingsurfaceofthetetheredhead,suggestingthattubulinbindingbythetetheredheadisinhibitedinthedwellstatebyparkingthetetheredheadagainsttheboundheadsoastomaskitstubulinbindingsite.
AlonsoetalSupplementaryFig.
S3.
CryoEMmapofthedwellstate(8)PageS7SAlonsoetalSupplementaryFig.
S4.
FittingthecryoEMmap.
Fig.
S4A|FrontViewDockingADockingBPageS8SFig.
S4B|SideViewDockingADockingBPageS9SFig.
S4C|TopViewDockingADockingBSupplementaryReferences1.
I.
Crevel,N.
Carter,M.
Schliwa,R.
Cross,EmboJ18,5863(1999).
2.
I.
M.
Crevel,A.
Lockhart,R.
A.
Cross,JMolBiol273,160(1997).
3.
A.
Lockhart,I.
M.
Crevel,R.
A.
Cross,JMolBiol249,763(1995).
4.
T.
Mitchison,M.
Kirschner,Nature312,232(Nov15-21,1984).
5.
A.
Davis,C.
R.
Sage,L.
Wilson,K.
W.
Farrell,Biochemistry32,8823(Aug31,1993).
6.
A.
Lockhart,R.
A.
Cross,EmboJ13,751(1994).
7.
J.
W.
Walker,G.
P.
Reid,J.
A.
McCray,D.
R.
Trentham,JAmChemSoc110,7170(1988).
8.
K.
Hirose,J.
Lowe,M.
Alonso,R.
A.
Cross,L.
A.
Amos,MolBiolCell10,2063(1999).
9.
K.
Hirose,E.
Akimaru,T.
Akiba,S.
A.
Endow,L.
A.
Amos,MolCell23,913(Sep15,2006).
10.
R.
Nitta,M.
Kikkawa,Y.
Okada,N.
Hirokawa,Science305,678(Jul30,2004).
11.
M.
Kikkawa,N.
Hirokawa,EmboJ(Aug31,2006).
tubulin
- www.sciencemag.org/cgi/content/full/316/5821/120/DC1相关文档
- assemblyakiba-online
- 周游akiba-online
- 补票akiba-online
- assessmentakiba-online
- ALTWakiba-online
- 2.2akiba-online
spinservers春节优惠:$149/月10Gbps圣何塞服务器-2*E5-2630Lv3 CPU,256G内存,2*1.6T SSD硬盘
spinservers是Majestic Hosting Solutions LLC旗下站点,商家提供国外服务器租用和Hybrid Dedicated等产品,数据中心包括美国达拉斯和圣何塞机房,机器默认10Gbps端口带宽,高配置硬件,支持使用PayPal、信用卡、支付宝或者微信等付款方式。农历春节之际,商家推出了几款特别促销配置,最低双路E5-2630Lv3机器每月149美元起,下面列出几款机器...
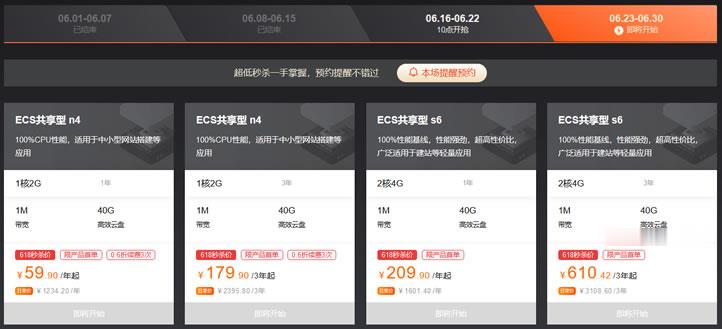
阿里云年中活动最后一周 - ECS共享型N4 2G1M年付59元
以前我们在参与到云服务商促销活动的时候周期基本是一周时间,而如今我们会看到无论是云服务商还是电商活动基本上周期都要有超过一个月,所以我们有一些网友习惯在活动结束之前看看商家是不是有最后的促销活动吸引力的,比如有看到阿里云年中活动最后一周,如果我们有需要云服务器的可以看看。在前面的文章中(阿里云新人福利选择共享性N4云服务器年79.86元且送2月数据库),(LAOZUO.ORG)有提到阿里云今年的云...
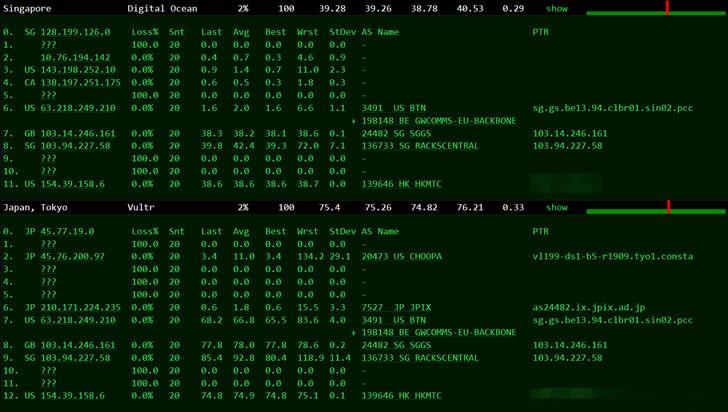
Megalayer新加坡服务器国际带宽线路测评
前几天有关注到Megalayer云服务器提供商有打算在月底的时候新增新加坡机房,这个是继美国、中国香港、菲律宾之外的第四个机房。也有工单询问到官方,新加坡机房有包括CN2国内优化线路和国际带宽,CN2优化线路应该是和菲律宾差不多的。如果我们追求速度和稳定性的中文业务,建议还是选择CN2优化带宽的香港服务器。这里有要到Megalayer新加坡服务器国际带宽的测试服务器,E3-1230配置20M国际带...
akiba-online为你推荐
-
Baby被问婚变绯闻黄晓明baby一起出来带娃,想要打破离婚传闻?杨紫别祝我生日快乐祝自己生日快乐内涵丰富的话比肩工场比肩成局 什么意思psbc.com怎样登录wap.psbc.comwww.qq530.com谁能给我一个听歌的网站?8090lu.com《8090》节目有不有高清的在线观看网站啊?www.vtigu.com如图所示的RT三角形ABC中,角B=90°(初三二次根式)30 如图所示的RT三角形ABC中,角B=90°,点p从点B开始沿BA边以1厘米每秒的速度向A移动;同时,点Q也从点B开始沿BC边以2厘米每秒的速度向点C移动。问:几秒后三角形PBQ的面积为35平方厘米?PQ的距离是多少www.5any.comwww.qbo5.com 这个网站要安装播放器www.789.com.cn有什么网站可以玩游戏的.sodu.tw给个看免费小说的网站