7.76aav.com
76aav.com 时间:2021-04-07 阅读:()
ORIGINALARTICLEDiseasecorrectionbycombinedneonatalintracranialAAVandsystemiclentiviralgenetherapyinSanlippoSyndrometypeBmiceCDHeldermon1,EYQin2,KKOhlemiller3,EDHerzog4,JRBrown5,CVogler6,WHou7,JLOrrock8,BECrawford5andMSSands2MucopolysaccharidosistypeIIIB(MPSIIIB)orSanlippoSyndrometypeBisalysosomalstoragediseaseresultingfromthedeciencyofN-acetylglucosaminidase(NAGLU)activity.
Wepreviouslyshowedthatintracranialadeno-associatedvirus(AAV)-basedgenetherapyresultsinpartialimprovementsofseveralaspectsofthedisease.
Inanattempttofurthercorrectthedisease,MPSIIIBmiceweretreatedat2–4daysofagewithintracranialAAV2/5-NAGLU(IC-AAV),intravenouslentiviral-NAGLU(IV-LENTI)orthecombinationofboth(BOTH).
TheBOTHgrouphadthemostcompletebiochemicalandhistologicalimprovementsofanytreatmentgroup.
ComparedwithuntreatedMPSIIIBanimals,alltreatmentsresultedinsignicantimprovementsinmotorfunction(rotarod)andhearing(auditory-evokedbrainstemresponse).
Inaddition,eachtreatmentgrouphadasignicantlyincreasedmedianlifespancomparedwiththeuntreatedgroup(322days).
Thecombinationarmhadthegreatestincrease(612days),followedbyIC-AAV(463days)andIV-LENTI(358days).
Finally,theBOTHgrouphadnearlynormalcircadianrhythmmeasureswithimprovementintimetoactivityonset.
Insummary,targetingboththesystemicandcentralnervoussystemdiseaseofMPSIIIBearlyinlifeappearstobethemostefcaciousapproachforthisinheritedmetabolicdisorder.
GeneTherapy(2013)20,913–921;doi:10.
1038/gt.
2013.
14;publishedonline28March2013Keywords:Sanlippo;behavioral;MPSIIIBINTRODUCTIONN-Acetyl-glucosaminidasedeciency(SanlippoSyndrometypeB,MucopolysaccharidosisIIIB)typicallycausesapediatriconsetdiseasecharacterizedphenotypically,byprogressivemotorandcognitivedeterioration,andhistologicallybyaccumulationoflysosomalinclusionsinmosttissues.
1Nocurrenttreatmentisapprovedinhumans.
Afterthegenewasidentied,2,3amurinemodelofmucopolysaccharidosistypeIIIB(MPSIIIB)wascreated.
4TheMPSIIIBmousesharesmanyofthebiochemical,histologicalandclinicalfeatureswiththehumandisease.
4,5SeveralgroupshavedemonstratedtheabilityofeitherintracranialorsystemicgenetherapyapproachestoreducelysosomaldistentioninthebrainsofMPSIIIBmice.
6–11Ourgroupalsodemonstratedimprovementsinhistologywithcorrespondingimprovementsinneurologicfunctionandlifespanusingintracranialgenetherapy.
12Intracranialdeliveryhasthusfardemonstratedthemostconsistentimprovementindiseaseprogression.
Increasesofapproximately30%inlifespanhavebeenobservedwithcentralnervoussystem(CNS)-directedtherapies.
AnintracranialgenetherapyapproachisnowbeingpursuedinalargeranimalmodelofMPSIIIB.
13Systemic-targetedgenetherapywasshowntoreducelysosomalstorageinperipheralorgans.
However,noneofthesesingleapproachescompletelyeradicatesintra-cytoplasmicinclusionsornormalizesthediseasephenotype.
WepreviouslyattemptedacombinationofCNS-directedgenetherapyandbonemarrowtransplantationwithlittleornobenetseenforthebonemarrowtransplantationarms.
However,thelevelofchimerismwasrelativelylowandtoxicitiesfromtheradiationconditioningwereevidentinthetransplantarm.
Inotherlysosomalstoragediseasemodels,therapiestotheCNSandtheperiphery,withenzymereplacement,bonemarrowtransplantationorgenetherapy,haveshowngreatlyimproveddiseasecorrectionespeciallywheninitiatedintheneo-natalperiod.
14–17Therefore,wehypothesizedthatneonatalcombinationtherapydirectedtoboththeCNSandtheperipherywouldprovidebettercorrectionofthedisease,especiallyifhighersystemiclevelsofN-acetylglucosaminidase(NAGLU)activitycouldbeattained.
Wedescribeherethebenetsobtainedfromeachmodeofgenetherapyandthesynergisticeffectofcombiningintracranialadeno-associatedvirus(AAV)-NAGLUandsystemiclentiviral-NAGLUgenetherapy.
RESULTSTreatmentsAllgenetherapyinjectionswereperformedinmicepupsat2–4daysofage.
IntracranialAAV-NAGLUtreatmentwas1DepartmentofMedicine,UniversityofFlorida,Gainesville,FL,USA;2DepartmentofInternalMedicine,WashingtonUniversity,StLouis,MO,USA;3DepartmentofOtorhinolaryngology,WashingtonUniversity,StLouis,MO,USA;4DepartmentofBiology,WashingtonUniversity,StLouis,MO,USA;5ZacharonPharmaceuticals,SanDiego,CA,USA;6DepartmentofPathology,SaintLouisUniversity,StLouis,MO,USA;7DepartmentofBiostatistics,UniversityofFlorida,Gainesville,FL,USAand8DepartmentofZoology,UniversityofWisconsin-Madison,Madison,WI,USA.
Correspondence:DrCDHeldermon,DepartmentofMedicine,UniversityofFlorida,1600SWArcherRoad,Box100278,Gainesville,FL32610,USA.
E-mail:coy.
heldermon@medicine.
u.
eduOrProfessorMSSands,DepartmentofInternalMedicine,WashingtonUniversityinStLouis,660SouthEuclid,CampusBox8007,StLouis,MO63110,USA.
E-mail:msands@dom.
wustl.
eduReceived14September2011;revised11February2013;accepted21February2013;publishedonline28March2013GeneTherapy(2013)20,913–921&2013MacmillanPublishersLimitedAllrightsreserved0969-7128/13www.
nature.
com/gtperformedasdescribedpreviouslywithsixdirectinjectionsof2mleachintofrontal,temporalandcerebellarregionsofthebrainofvectorataconcentrationof1.
51012viralparticlesperml.
5,12Intravenouslentiviral-NAGLUinjectionswerealsoperformedasdescribedpreviouslybyinjectionof100mlof1.
6108infectiousunitspermlviralaliquotintothesupercialtemporalvein.
18NAGLUactivityBiochemicalanalysisofN-acetyl-glucosaminidaseactivityforvariousorganswasdeterminedinmicefromeachgroupatB8monthsofageandcomparedwithuntreatedMPSIIIBanimals(MPSIIIBNOTX;Figure1).
TheMPSIIIBanimalsreceivingonlyintravenouslentiviralvectortreatment(MPSIIIBIV-LENTI)haddetectableNAGLUactivityinallorgansassayed(o2%ofnormalinthebrain(Po0.
05)andkidneys(notsignicant(NS)),11%inheart(Po0.
01),12%inlungs(NS),34%inliver(Po0.
001),34%inspleen(Po0.
05)and28%intheserum(NS;datanotshown).
Conversely,animalstreatedonlywithintracranialAAV(MPSIIIBIC-AAV)hadapproximately200%(Po0.
05),3%(NS)and5%(NS)ofnormalactivityinthebrain,liverandserum(datanotshown),respectively,andlittleornoactivityinthespleen,heart,lungsorkidneys.
Combinationtherapy(MPSIIIBBOTH)yieldedNAGLUactivitylevelsof424%forbrain(Po0.
01),13.
6%forliver(Po0.
001),6.
7%forheart(Po0.
01),19.
8%forspleen(Po0.
01),2.
9%forlungs(Po0.
05),42%forserum(NS)ando1%inthekidneys(NS).
Normalmicetreatedwithboththerapies(NORMALBOTH)hadnosignicantchangeinNAGLUactivityfromnormalmiceforthevisceralorgans(P40.
05foreverycomparison).
SecondaryenzymeactivityInmostlysosomalstoragedisorders,otherlysosomalenzymes,suchasb-glucuronidase(GUSB),areelevated.
TheresolutionofFigure1.
NAGLUactivitywasmeasuredinbrain,liver,heart,spleen,kidneysandlungsfromuntreatedandtreatedMPSIIIBmice.
Meanactivitylevelss.
e.
m.
foreachgrouprelativetotheNormalnotreatment(NOTX)grouparedepictedinthegraphs.
BOTH,bothIV-LENTIandIC-AAVtreatments;IC-AAV,intracranialadeno-associatedviralNAGLUvector;IV-LENTI,intravenouslentiviralNAGLUvector.
*Po0.
05;**Po0.
01;***Po0.
001comparedwithMPSIIIBNOTXgroup.
CorrectionofSanlippoSyndrometypeBCDHeldermonetal914GeneTherapy(2013)913–921&2013MacmillanPublishersLimitedthissecondaryelevationhasbeenusedasasurrogatefortherapeuticresponse.
SecondaryelevationsofGUSBactivityinthevariousgroupsaredepictedinFigure2andstatisticallycomparedwiththeMPSIIIBNOTXgroup.
UntreatedMPSIIIB(MPSIIIBNOTX)animalshadsignicantelevationsinGUSBcomparedwithnormalmiceforallorgansassayed:brain(358%,Po0.
001),liver(175%,Po0.
01),spleen(343%,Po0.
05),heart(619%,Po0.
05),lungs(645%,Po0.
001)andkidneys(439%,Po0.
001).
IC-AAVmicehadsignicantreductions(nearlynormal)insecondaryelevationscomparedwithuntreatedMPSIIIBmiceinthebrain(Po0.
0001),387%normalinthelungs(Po0.
01),butnosignicantreductionsintheothervisceralorgans.
IV-LENTI-treatedmicehadsignicantreductionsinGUSBactivityinthebrain(228%,Po0.
01),kidneys(217%,Po0.
05),andlungs(227%,Po0.
0001).
Combinationtreatmentresultedinnormalizationoflevelsinthebrain(95%,Po0.
0001),andsignicantreductionsinthekidneys(188%,Po0.
01)andlungs(183%,Po0.
0001).
GUSBactivityinthespleenandheartwerereducedinthecombinationarmbutdidnotreachsignicance(P0.
052and0.
099,respectively).
Thecombination-treatedwild-typeanimalsremainedwithinthenormalrangeexceptforthebrain,withasignicantreductionto82%ofnormal.
HistologyMPSIIIBBOTHmicehadthebestresponseintheCNS,withmarkedreductioninlysosomalstorageinglialandmeningealcellsaswellasincorticalneuronsasdepictedinFigure3.
ThesesamecelltypesinMPSIIIBIC-AAVmicehadalessstrikingbutdeniteresponsetotheintracranialtherapy.
MPSIIIBIV-LENTImicehadnoresponseinneuronsandonlymildreductioninstorageinglialandmeningealcells(Table1).
ThespleenandliverintreatedmiceFigure2.
Secondaryelevationofb-glucuronidaseactivityinbrain,liver,heart,spleen,kidneysandlungsfromuntreatedandtreatedMPSIIIBmice.
Meanactivitylevelss.
e.
m.
foreachgrouprelativetotheNormalNOTXgrouparedepictedinthegraphs.
GroupdesignationsareidenticaltothoseinFigure1(*Po0.
05,**Po0.
01,***Po0.
001,****Po0.
0001comparedwithMPSIIIBNOTXgroup).
CorrectionofSanlippoSyndrometypeBCDHeldermonetal915&2013MacmillanPublishersLimitedGeneTherapy(2013)913–921showedminimalornoresponsewithIC-AAVtherapyonly(Table1).
However,therewasaclearandsimilarreductioninstorageinthesesitesinboththeIV-LENTIandBOTHgroups.
Asagroup,themicetreatedwithcombinedIC-AAVandIV-LENTItherapyhadthebestresponsewiththegreatestreductioninstorage.
Glycosaminoglycan(GAG)analysisInordertoassessthelysosomalstoragemorequantitatively,theSensi-ProNon-ReducingEnd(NRE)assaywasperformedonbrainhomogenatesofadultmicefromeachgroupinordertomeasurethepathologicGAG(pGAG)accumulationofheparansulfate.
19–21Figure4depictsthereductioninbrainheparansulfatebyeachtreatment.
ComparedwiththeMPSIIIBNOTXgroup,MPSIIIBIV-LENTIhadnosignicantreductioninpGAG(85%ofNOTX,PNS).
MPSIIIBIC-AAV(24%ofNOTX,Po0.
01)andMPSIIIBBOTH(18%ofNOTX,Po0.
01)hadsignicantlyreducedpGAG.
NormalmicehadnodetectablepGAG.
CircadianactivityLong-termrunningwheelrecordingsfrom14to24weeksofagewereperformedwithmicefromeachexperimentalgroupundera12-hlight:12-hdarkcycle.
WepreviouslydescribedadifferencebetweenuntreatedMPSIIIBandnormalanimalsintwocircadianmeasurements:percentageofactivityoccurringduringthelightphase(greaterinMPSIIIB)andthetimefromlightsofftoactivityTable1.
LysosomalstorageinbrainandviscerabylightmicroscopyinMPSIIIBmiceaftertreatmentwithintracranialAAV,intravenouslentiviralorcombinedgenetherapyTissueIC-AAV(n4)IV-Lenti(n4)BOTH(n3)LiverHepatocytesNCNC-kNC-kkKupffercellsNC-kk-kkk-kkSpleenSinusliningcellsNC-kkk-kkkkkkBrainCorticalneuronsNC-kkNCkk-kkkMeningesk-kkkkkkGliakk-kkkNC-kkkkAbbreviations:IC-AAV,intracranialadeno-associatedvirus/5-N-acetylglu-cosaminidase;IV-LENTI,intravenouslentiviral-N-acetylglucosaminidase;MPSIIIB,mucopolysaccharidosistypeIIIB;NC,nochangeinlysosomalstoragefromage-matcheduntreatedMPSIIIBmouse.
k,Slightreductioninstorage;kk,moderatereductioninstorage;kkk,markedreductioninstorage,allcomparedwithage-matcheduntreatedMPSIIIBmouse.
Figure3.
Lysosomalinclusionsinparietalcortex.
Representativesectionsofparietalcortexof(a)MPSIIIB,(b)MPSIIIBIV-LENTI,(c)MPSIIIBIC-AAV(d)MPSIIIBBOTHmiceareshown.
Blackarrowsindicateneuronallysosomalinclusionsandwhitearrowsindicatelysosomaldistensioninglialcells.
Figure4.
Heparansulfatelevelsinthebrainofuntreatedandtreatedmice.
Pathologicglycosaminoglycan(pGAG)levelsinbrainhomogenatess.
e.
m.
foreachgrouparedepictedinpgpermgofprotein(N4–8foreachgroup,NS—notsignicant,**Po0.
01,****Po0.
0001comparedwiththeMPSIIIBNOTXgroup).
CorrectionofSanlippoSyndrometypeBCDHeldermonetal916GeneTherapy(2013)913–921&2013MacmillanPublishersLimitedonset(shorterinMPSIIIB).
5Similardifferenceswereobservedinthecurrentstudy,however,percentactivityduringthelightphasedidnotreachstatisticalsignicance(gurenotshown)aftercorrectionformultiplecomparisonsbetweentheMPSIIIBNOTX(mean15.
14%)andtheNormalNOTXgroup(mean9.
60%;P0.
14).
AlthoughtheaverageactivityoftheMPSIIIBIC-AAV(mean14.
23%)andMPSIIIBBOTH(mean12.
03%)groupswereincreasinglynumericallyimprovedfromtheMPSIIIBNOTXgroupandclosertotheNormalNOTXgroup,theywerenotstatisticallysignicantlydifferentaftercorrectionformultiplegroups.
Thetimeofdailyactivityonset(phaseangleofentrainment)wassignicantlyearlierintheMPSIIIBNOTXanimalscomparedwithNormalNOTXmice(P0.
007;Figure5).
TherewasnodifferenceinthephaseangleofentrainmentbetweentheMPSIIIBNOTXandtheMPSIIIBIV-LENTIgroups.
However,theMPSIIIBIC-AAVtrendedtowardimprovement(P0.
078)andMPSIIIBBOTHwassignicantlyimprovedrelativetotheuntreatedMPSIIIBmice(P0.
003).
TheMPSIIIBBOTHgroupphaseangleisnotdifferentthantheNormalNOTX(P0.
710)UntreatedMPSIIIBandnormalanimalshadnodifferenceintotaldailyactivitylevelsimilartopublishedresults.
5NoothertreatmentgroupwasdifferentthannormalorMPSIIIBuntreatedmicefortotalactivity.
Thecombination-treatednormalanimalswerenodifferentthanthenormaluntreatedanimalsforanycircadianparametertested.
AuditoryfunctionAuditory-evokedbrainstemresponsethresholdswereperformedoneachgroupatapproximately8–8.
5monthsofage.
Similartoourpreviousndings,MPSIIIBNOTXmicehadsignicantlydiminishedhearingcomparedwithnormalanimals,reachingthemaximumsoundoutputleveloftheequipmentatseveralfrequencieswithoutgeneratingaresponse(Figure6).
EachoftheMPSIIIBtreatmentgroupshadaconsistentlylowerresponsethresholdatallfrequenciestestedcomparedwithMPSIIIBNOTXmice.
Basedonagroup-wisecomparisonofthetreatmentgroups,thereappearedtobeatrendtowardslowerthresholdsintheIC-AAVgroupcomparedwiththeIV-LENTIgroup.
MPSIIIBBOTHmiceweresignicantlyimprovedcomparedwitheitherIC-AAVorIV-LENTImicebutremainssignicantlydifferentthantheNormalNOTXgroup(Po0.
001).
Surprisingly,theNormalBOTHanimalsalsohaddiminishedhearingcomparedwithNormalNOTXanimals.
Thispresumablyreectssomeadverseeffectofvirusadministrationonhearing,buttheNormalBOTHanimalsstillhaddemonstrablybetterhearingthresholdsthanMPSIIIBmice.
MotorfunctionWepreviouslydemonstratedIC-AAVtreatmentcoulddelaytheprogressionofmotordecitsinMPSIIIBmiceasmeasuredusingarockingrotarod.
Inthecurrentstudy,wedemonstratethateachofthetreatmentinterventionssignicantlydelayedtheprogressionofmotordysfunctioncomparedwithMPSIIIBNOTXanimals(Figure7).
Themediantimetoalatencyofp60sontherodincreasedfromB40weeksinMPSIIIBNOTXanimalstoB78weeksinMPSIIIBBOTHmice.
MPSIIIBIC-AAVmicereachedthemedianlatencyatB62weeksandMPSIIIBIV-LENTImicereachedthatlatencyatB52weeks.
ThetimefortheMPSIIIBBOTHmicetoreachthemedianlatencywassignicantlygreaterthananyotherMPSIIIBgroup.
ThemedianhadnotbeenreachedforeitherNormalgroupat90weeksofageandisstatisticallylongerthantheMPSIIIBBOTHgroup(P0.
05).
Figure6.
HearingsensitivityindecibelsoftreatedanduntreatedMPSIIIBandNormalmice.
Auditory-evokedbrainstemresponsesrecordingwasperformedat5,10,20,28.
3and40kHzon9–10animalspergroupat8–9monthsofage.
Eachgroup'smeandecibelthresholdtodetecttheaudiblestimulis.
d.
isshown.
AlltreatmentgroupsareimprovedwhencomparedwithMPSIIIBNOTXgroupoverallfrequencies(Po0.
001byrepeatedmeasureANOVA).
Figure5.
Timeofactivityonset.
Meantimesofdayofactivityonsetareplotteds.
e.
m.
Lightoffsetat1900hoursisindicatedwithgreyshading.
NotethatuntreatedMPSIIIBmiceandMPSIIIBmicetreatedwithlentivirusstartedtheirdailyrunningabout15minbeforelightsoff,whereasMPSIIIBmicetreatedwithAAVorbothlentivirusandAAVstartedrunningabout30minafterlightsoff,similartonormal,untreatedmice(n6foreachgroup,**Po0.
01comparedwithMPSIIIBNOTXgroup).
Figure7.
RockingrotarodperformanceLog-Rankanalysis.
Alatencyof60sorlesswasusedasathresholdtorepresentmotordysfunctionintheLog-Rankanalysisthatisgraphed.
Allgroups(n6–10)aresignicantlylongerthanMPSIIIBNOTX.
MPSIIIBBOTHissignicantlylongerthanIC-AAV(Po0.
05)andIV-LENTI(Po0.
001).
ThereisnodifferencebetweenNormalNOTXandNormalBOTHgroupssothelatterisnotshownforgureclarity(**Po0.
01,***Po0.
001comparedwithMPSIIIBNOTXgroup).
CorrectionofSanlippoSyndrometypeBCDHeldermonetal917&2013MacmillanPublishersLimitedGeneTherapy(2013)913–921LifespanWeshowedpreviouslythatIC-AAVtreatmentprolongsthelifeofMPSIIIBanimalsby4100days.
WendasimilarincreaseinmedianlifespanofIC-AAVinthisstudy(Figure8).
AlltreatmentgroupsdemonstrateasignicantincreaseinmediansurvivalrelativetoMPSIIIBNOTX(322days).
MPSIIIBIV-LENTImediansurvivalincreasedto358days,MPSIIIBIC-AAVto452daysandMPSIIIBBOTHto612days.
TherearenoapparentdifferencesinsurvivalbetweentheNormalandNormalBOTHgroupsupto720daysofage(datanotshown),andmediansurvivalhasnotbeenreachedinthesegroups,andissignicantlylongerthananyMPSIIIBtreatmentgroup.
AdverseeventsWehadtheopportunitytoexamineatleastfourmicefromallgroupsatB280daysofageandtwoMPSIIIBIC-AAV,twoMPSIIIBBOTHandnineNormalBOTHmicefrom588–726daysofagefortumorformation.
Noneofthemiceexaminedhaddemonstrabletumorsongrossexamination.
DISCUSSIONThemostimportantmeasuresofclinicalbenetforanytherapyarequalityoflifeandquantityoflife.
Wehavechosentheneonataltimeframefortreatmentbecauselysosomalstoragediseasetreatmentbybonemarrowtransplantinhumanshasdemonstratedthattheearliestpossibletreatmentmustbepursuedtopreventirreversibleneurologicdamage.
Thisislikelytobethemostefcaciousinterventiontimeframeinhumansoncenewbornscreeningforthisdisorderbecomescommonplace.
Wehavedemonstratedthatneonataltreatmentwitheitheranintravenousinjectionofalentiviralvector,intracranialinjectionofanAAVvectororthecombinationofbothcansignicantlyimprovesurrogatesforqualityoflife(motorfunction,hearingandcircadianrhythm)andincreaselifespan.
Inallcases,thesystemicapproachwaslessefcaciousthantheintracranialapproach.
Thisisnotsurprisinggiventhepredominantlyneurologicmanifesta-tionsofMPSIIIB.
However,thehumandiseasehasmanifestationsthatarenotintheCNS,suchashepatomegaly,hernias,diarrheaandearinfectionswitheffectsonhearingthatmaybeacombinationofcentralandperipheralneurologiceffectsandearstructureabnormalities.
22–26Inaddition,someofthebenetintheIV-LENTIgroupmayberelatedtotheverysmallamountofactivityobservedinthebrainsoftheseanimals,presumablefromasmallamountoflentiviraltransductionacrosstheblood–brainbarrierashasbeenseenbyotherinvestigators.
27Thismaybesupportedbytherelativelysmalldecreaseinbrainheparansulfateinthisgroup,whichwasnotstatisticallysignicant.
WealsocannotruleoutthepossibilitythatgreaterbenetmightbeachievablefromthesystemicapproachifweobtainedhigherexpressionofNAGLUbyeitherusingahigherorrepeateddoseofvector.
Althoughwewereabletoattainsupra-normallevelsinthebrainwiththecombinationofsystemicandintracranialtreatments,thelevelsofNAGLUactivitywerelessthanhalfnormalinallotherorgansassayed.
Interestingly,inourstudy,thecombinationapproachconsistentlyyieldedlowerorganactivitythanthesystemicmonotherapyandhigherbrainactivitythantheCNS-directedmonotherapy.
Thismaybeduetodisruptionoftheblood–brainbarrierduringtheCNSinjectionsallowingsystemicvectortoshunttothebrainreducingtheotherorganviralexposure.
WeattemptedtodeterminetherelativecontributionoflentiviralandAAVtransductioninthebrain.
However,becauseofthepresenceofrepetitiveelements,identicalinsertsandpoorDNAqualityafterhomogenizationneitherthequantitativePCRnorinsituhybridizationapproachestoquantifyAAVversuslentivirusweresuccessful.
WecannotruleoutthepossibilitythatthecombinationapproachanimalsreceivedagreatervectorcopynumberthantheIC-AAVgroup,despitethesameinjectionvolumefromthesamelotofvirus,thatcouldexplainthedifferencesbetweenthegroups.
Weobservedthatsomeorgans,suchaslungs,heartandkidneysseemmoreresistanttotransductionashasbeenobservedbyothergenetherapyinvestigations,27butstillhavesubstantialreductionofsecondaryenzymeelevation.
Thisabrogationofsecondaryelevationmaybethroughlow-levelNAGLUenzymecrosscorrection,frommorehighlytransducedorganssuchastheliverorspleen.
However,thislow-levelcrosscorrectionisnotenoughtomarkedlyreducelysosomalinclusionshistologically.
Perhapsthelevelofcrosscorrectionneededtoreducesecondaryenzymeelevationsislowerthanwhatisneededtomarkedlyreducelysosomaldistention.
Thishasbeenobservedwiththeliver-directedgenetherapyforMPSVIIaswell.
17Theadverseeffectsonauditory-evokedbrainstemresponsethresholdsfollowingvirusadministrationintheNormalgroupmostlikelydidnotresultfromAAVasthisgroupwasnotadverselyaffectedinourpriorstudy,whichevaluatedICAAVgiveninthesamemanner.
WhethertheadverseeffectsareattributabletoadirecteffectoflentivirusorrathertoxiceffectsofexcessNAGLUtonormalhearingisnotyetknown.
However,thiscouldbeaddressedbythedeterminationoflong-termeffectsonhearingstructureandfunctionaftersystemiclentiviraltreatmentofNormalmicewitheitherNAGLUorareporterenzyme.
Asweoriginallyhypothesized,thecombinationapproachappearstohavethegreatestbenet.
AlthoughMPSIIIBisasimplemonogenicdisease,ithasacomplexbiochemical,histologicalandclinicalphenotype.
AsNAGLUisexpressedinvirtuallyeverycellofthebody,mosttissueshavesomelysosomalstorage.
Consequently,multipletissuesmustbetreatedtoprovidethemaximumbenet.
Interestingly,thecombinationtherapyappearstobeadditiveforsomemeasuresthataffectqualityoflifesuchashearingandmotorfunction,andsynergisticformeasuresofcircadianfunction(differenceintimetoactivityonsetof2minwithIV-LENTI,22minwithIC-AAVand38minwithBOTH)andoverallsurvival(differenceinmediansurvivalof36dayswithIV-LENTI,130dayswithIC-AAVand290dayswithBOTH).
Notsurprisingly,thecombinationtreatmentwasnotbestedbyeithersingletherapyinanyfunctionalassessment.
Unfortunately,despitelong-termfunctionalimprovementsandsubstantialbenetsinsurvivalofthecombinationapproach,theprogressionofdiseaseduringthelastseveralweeksoflifeforeachoftheFigure8.
Survival.
AlltreatedmicewereanalyzedformediansurvivalbyKaplan–Meieranalysisandplotsareshown.
AlltreatedgroupshadsignicantlyimprovedsurvivalcomparedwithMPSIIIBNOTX.
MPSIIIBBOTHissignicantlylongerthanIC-AAV(Po0.
05)andIV-LENTI(Po0.
001).
ThereisnodifferencebetweenNormalNOTXandNormalBOTHgroupssothelatterisnotshownforgureclarity(**Po0.
01,***Po0.
001comparedwithMPSIIIBNOTXgroup).
CorrectionofSanlippoSyndrometypeBCDHeldermonetal918GeneTherapy(2013)913–921&2013MacmillanPublishersLimitedgroupsremainedsimilarwithpoorcoatcare,urinaryretentionandlossofbalance.
Thecombinationtherapyapproachusingtwodifferentvectorsinthecurrentstudyisnottheonlydualapproachthatcanbeenvisioned.
Weselectedalentiviralvectorforthesystemicapproachforitsefcacyatproducingsustainedexpressionofaproteinproductandthelackofknownimmuneinactivationinhumantrials.
28–30AhighersustainedlevelofNAGLUproductionmayhavebeenobtainedwithlentiviraltransducedbonemarrowselectedforhighenzymelevelashasbeendemonstratedbyothers.
31Alternatively,asystemicAAV9vectorapproachmayyieldsimilarsystemicbenetswithasingleperipheralinjectionsite,32however,thisserotypehasnotyetbeenusedinhumans.
Incontrasttolentivirustrials,humansystemicAAVgenetherapyapproacheshavebeenlimitedbyimmuneresponsestothevirus-infectedcells.
ThesystemicAAVapproachneedsfurtherstudytoavoidimmuneinactivationthathasbeenseeninhumantrials.
33,34Inadditiontodeterminingtheefcacyofadualgenetherapyapproach,thesafetyofAAVandlentiviralvectorsrequiresadditionalstudy.
Severalrecentstudieshavedemonstratedthelong-termsafetyofsystemicdeliveryofAAVvectorsinbothrodentsandnon-humanprimates.
35,36However,anumberofstudieshavealsoshownanincreaseintumorigenesisinAAV-treatedanimals.
37–42Givenrecentndingsregardingrecombinantlentiviralintegrationeventsnearactivegenes,36itseemsprudenttomonitorfortumorformationinfuturepre-clinicalexperimentsandhumantrials.
Wehavenotobservedanyhepatocellularcarcinomaorothermalignanciesinthecurrentstudydespitelongfollow-up.
Finally,othercombinationapproachescouldalsoprovetobeefcacious.
43Severalstudieshaveshownthatcombininghematopoieticstemcell-mediatedtherapywithgenetherapyorsubstratereductiontherapygreatlyincreasestheefcacyinthemurinemodelofKrabbedisease16,44andmetachromaticleukodystrophy.
45Itwasalsoshownthattheadditionofsmall-moleculeanti-inammatoryorsubstratereductionagentsenhancedtheefcacyofbothneuronalstemcell-andhematopoieticstemcell-mediatedtransplantationinthemurinemodelofSandhoffdisease.
46,47Thecaveattothisapproachistherelativelyhighmorbidityandmortalityofstemcelltransplantmethodsinhumansfromtheconditioningregimens,infections,andforallogeneictransplantsfromgraft-versus-hostdisease.
Inconclusion,combinationneonatalintracranialandsystemicNAGLUgenetherapyprovidessignicantandclinicallymean-ingfultherapeuticbenetinamousemodelofMPSIIIB.
However,studyofadditionalinterventionsiswarrantedasthecurrentstrategyisnotcompletelycorrective.
MATERIALSANDMETHODSViralconstructsAAV-NAGLUwasconstructedaspreviouslydescribedwithahuNAGLUcDNA(giftofElizabethNeufeld),AAV2genome,CMVenhancer,chickenb-actinpromoter,SV40poly-Asignaland30untranslatedregionfromtherabbitb-globingene.
VectorwasproducedattheUniversityofFloridaVectorCorewithapseudotypeAAV5capsid,anddilutedto1.
51012viralparticlespermlwithlactatedRinger'ssolutionbeforefreezingat851C.
Lentiviral-NAGLUwasconstructedwiththesamehuNAGLUcDNAintotheMNDvectorplasmid(kindgiftfromDKohn)withadelta-gag,centralpolypurinetractandthemyeloproliferativesarcomavirusenhancer,negativecontrolregiondeleted,Dl587revprimer-bindingsite-substituted(MND)promoter,SV40poly-Atailanddeltau330longterminalrepeat.
VectorwasproducedintheSands'labwithafour-plasmidsystemin293Tcellsandconcentratedto1.
6108infectiousunitspermlbeforealiquotingandfreezingat851C.
MiceC57BL/6NAGLU-decientmice(kindgiftfromENeufeld)weremaintainedbystrictsiblingmatingbyMSSatWashingtonUniversitySchoolofMedicine.
GenotypingwasdoneontissueofnewbornmicebyenzymeassayorNAGLUexon6andneomycininsertioncassettePCR.
AllproceduresonanimalswereinaccordancewiththeGuidelinesofInstitutionalAnimalCareandUseCommitteeatWashingtonUniversityinStLouis.
TreatmentsAt2–4daysofageallmicewereallocatedtotreatmentgroups:untreatedMPSIIIB(MPSIIIBNOTX,n19,15males),MPSIIIBtreatedwithintracranialAAV-NAGLU(MPSIIIBIC-AAV,n19,10males),MPSIIIBtreatedwithintravenouslentiviralNAGLU(MPSIIIBIV-LENTI,n19,7males),MPSIIIBtreatedwithbothintracranialAAV-NAGLUandintravenouslentiviralNAGLU(MPSIIIBBOTH,n16,9males),normaluntreated(NormalNOTX,n15,10males)andnormalwithbothintracranialAAV-NAGLUandintravenouslentiviralNAGLU(NormalBOTH,n17,8males).
Allgenetherapyinjectionswereperformedinmicepupsat2–4daysofage.
IntracranialAAV-NAGLUtreatmentwasperformedasdescribedpreviouslywithsixdirectinjectionsof2mleachintofrontal,temporalandcerebellarregionsofthebrainusinga32-Gneedle.
5,12Intravenouslentiviral-NAGLUinjectionswerealsoperformedasdescribedpreviouslybyinjectionof100mlofviralaliquotintothesupercialtemporalvein.
18Whencombined,theAAVinjectionswereperformedrstandthesystemicinjectionswereperformedwithin5–60min.
HistologyandbiochemistryMicefromeachgroup(n3–8forMPSIIIBNOTXandMPSIIIBBOTH,andn4–8allothergroups)between242and259daysofagewerekilledbyCO2asphyxiation.
Liver,spleen,kidneys,heart,lungsandbrainwereharvested.
Partofeachorganwasimmersionxedin2%gluteraldehyde/4%paraformaldehydeinphosphate-bufferedsalineandpartwasashfrozeninliquidnitrogenandstoredat851Cuntilmechanicalhomogenizationin10mMTris(pH7.
5),150mMNaCl,1mMdithiothreitoland0.
2%TritonX-100.
FixedtissuewasembeddedinSpurr'sresinand1-mm-thicksectionswerestainedwithtoluidinebluebeforeblindedevaluationoflysosomalstorageandvacuolization.
CelldebriswaspelletedandsupernatantswerecollectedforenzymeassaysofNAGLUandGUSBactivity.
DuplicateNAGLUassayswereperformedusing20mlofsupernatantaddedto40mlof0.
2mM4-methylumbelliferone-N-acetyl-a-D-glucopyranoside(Sigma,StLouis,MO,USA),0.
1MNaC2H3O2,0.
5mgml1bovineserumalbuminandincubatedat371C.
48Reactionswerestoppedwith1mlof0.
2MNa2CO3,0.
32Mglycine.
Substratecleavagewasdeterminedatexcitation365nmandemission448nmusingaHitachiF-2000FlourescenceSpectrophotometerusingastandardcurveof0.
5–5nmml1.
Specicactivitywascorrectedforproteinconcentration.
DuplicateGUSBassayswereperformedsimilarlyusingthe4-methylumbelliferoneenzymeassaymethodpreviouslydescribed.
49Activitylevelswereanalyzedbyone-wayanalysisofvariance(ANOVA)forcomparisonoftreatmentstoNOTXgroupsafterconrmationofadifferencebetweenNORMALandMPSIIIBNOTXgroupsbyStudent'st-test.
Brown-ForsytheTestofequalvarianceswassatisedasnotsignicantfororgancomparisonsofbrain,liver,heart,lungs,spleenandkidneysofGUSB.
GAGanalysisHomogenizedbrainsamples(N4–8foreachgroup,allfrommiceolderthan200days)werecodedwithanumericalidentierandsentblindedtoZacharonPharmaceuticalsInc.
(SanDiego,CA,USA)forpGAGanalysisusingSensi-ProNREassay.
pGAGaretheGAGfragmentspresentduetothedeciencyofthespeciclysosomalenzyme.
TheSensi-ProNREassayisahighlyspecicandsensitiveassaythatuseshigh-performanceliquidchromatographytoquantitatethereductioninlysosomalGAGaccumula-tion,bylabelingandquantifyingtheNREsoftheseGAGfragments.
InMPSIIIB,thepGAGmarkersareuniqueNRE-derivedtrisaccharidesthatterminateinN-acetylglucosamineduetothelysosomaldeciencyinN-acetylglucosaminidaseasdescribedindetailbyLawrenceetal.
20,21TheGAGswereextractedandpuriedbydiethylaminoethanol(DEAE)chromatography,digestedwithheparinlyases,uorescentlylabeledandanalyzedaspreviouslydescribed.
19One-wayANOVAwasusedtocomparepicogramsofpGAGpermicrogramofproteinforeachtreatmentgrouptotheMPSIIIBNOTXgroup.
CircadianassessmentsSixmalemicefromeachgroupwerestudiedfrom14to24weeksofageaspreviouslydescribed.
5AllmicewerehousedindividuallyincageswithaCorrectionofSanlippoSyndrometypeBCDHeldermonetal919&2013MacmillanPublishersLimitedGeneTherapy(2013)913–921runningwheelwithinlight-tightventilatedchambersilluminatedbyuorescentbulbs(F30T12-SP41-RS,GeneralElectric,Faireld,CT,USA,3.
91017to6.
91018photonss1m2)atthebottomofthecages.
Wheelrunningactivitywasrecordedin1minbins(Clocklab,Actimetrics,Evanston,IL,USA),whereasmicewereexposedtoalight–darkschedule(lightsonat0700andoffat1900hours).
Weanalyzedphaseangleofentrainment(delaybetweendailylightoffsetandonsetofactivity),totaldailyactivityandproportionofdailyactivityinthelightphaseofthephotocycleusingClocklab.
50Statisticalanalysiswasbyrepeated-measuresANOVA.
AuditoryevaluationAuditory-evokedbrainstemresponseswereperformedat8–8.
5monthsofageusingproceduressimilartothosedescribedpreviously.
5,12Micewereanesthetizedwithketamine/xylazine(85/15mgkg1,i.
p.
)whilecoretemperaturewasmaintainedat37.
0±1.
01Cbyathermostaticallyregulatedheatingpadmonitoredviaarectalprobe(YellowSprings).
Platinumneedleelectrodes(Grass,WestWarwick,RI,USA)wereplacedsubcutaneouslyintheback,vertexandbehindtherightearwhileconnectingtoaGrassP15differentialamplier(100–10000Hz,100).
ACambridgeElectronicDesignMicro1401(CambridgeElectronicDesign,Cambridge,UK)runningSIGNALandcustomaveragingsoftwaredigitizedthesignalat30kHz.
Toneburststimuliat5,10,20,28.
3and40kHzweredelivered1000timesat20s1usinganAlpineSPS-OEOAcoaxialspeaker(Crutcheld,Charlottesville,VA,USA)located10cmlateraltotherightear.
Ateachtestfrequency,thestimuluslevelwasreducedin5dBminimumstepstodeterminetheminimumsoundpressurelevelrequiredforvisualdetectionofWaveI.
Repeated-measureANOVAwithDunnetsmultiplecomparisoncorrectionwasperformedtocompareeachtreatmentgrouptotheMPSIIIBNOTXcontrol.
Separateone-wayANOVAwasperformedfordatafromallgroupsateachtestfrequency,followedbyBonferronimultiplecomparisontests.
Samplesizesbygroupwereasfollows:MPSIIIBNOTX,n10;MPSIIIBIC-AAV,n9;MPSIIIBIV-LENTI,n9;MPSIIIBBOTH,n10;NormalNOTX,n10;NormalBOTH,n10.
MotorfunctionassessmentAswedescribedpreviouslyinthismodel5,12miceweretrainedonarotarod(UGOBasile,Varese,Italy)movingataspeedof10r.
p.
m.
thatswitcheddirectionaftereachfullrotationforupto180sperattempt,withthreeattemptsperday.
Testsweredoneevery28daysfrom196upto672daysofage(MPSIIIBNOTX,n6;MPSIIIBIC-AAV,n9;MPSIIIBIV-LENTI,n10;MPSIIIBBOTH,n10;NormalNOTX,n9;NormalBOTH,n8).
Longestlatencytofallfromtherotarodofthethreeattemptswasusedforcomparisons.
DatawereinterpretedusingaLogRanktestfortimetolatencyofo60sonrotarod.
LifespanAlltreatedanimalswereanalyzedbyintentiontotreatwithaGehan–Wilcoxontest.
Kaplan–Meiercurvesweregeneratedtoassesstheeffectoftreatmentsonsurvival.
CONFLICTOFINTERESTJillianRBrownandBrettECrawfordarefull-timeemployeesandshareholdersofZacharonPharmaceuticals.
Allotherauthorshavenoconictofinteresttoreport.
ACKNOWLEDGEMENTSThisworkwasfundedinpartbyNIHgrantsNS043205(MSS),HD055461(MSS),K08DK085141-01(CDH)andbytheSanlippoChildren'sResearchFoundation(CDH).
REFERENCES1YogalingamG,HopwoodJJ.
MoleculargeneticsofmucopolysaccharidosistypeIIIAandIIIB:diagnostic,clinical,andbiologicalimplications.
HumMutat2001;18:264–281.
2WeberB,BlanchL,ClementsPR,ScottHS,HopwoodJJ.
CloningandexpressionofthegeneinvolvedinSanlippoBsyndrome(mucopolysaccharidosisIIIB).
HumMolGenet1996;5:771–777.
3ZhaoHG,LiHH,BachG,SchmidtchenA,NeufeldEF.
ThemolecularbasisofSanlipposyndrometypeB.
ProcNatlAcadSciUSA1996;93:6101–6105.
4LiHH,YuWH,RozengurtN,ZhaoHZ,LyonsKM,AnagnostarasSetal.
MousemodelofSanlipposyndrometypeBproducedbytargeteddisruptionofthegeneencodingalpha-N-acetylglucosaminidase.
ProcNatlAcadSciUSA1999;96:14505–14510.
5HeldermonCD,HennigAK,OhlemillerKK,OgilvieJM,HerzogED,BreidenbachAetal.
Developmentofsensory,motorandbehavioraldecitsinthemurinemodelofSanlipposyndrometypeB.
PLoSOne2007;2:e772.
6FuH,SamulskiRJ,McCownTJ,PicornellYJ,FletcherD,MuenzerJ.
NeurologicalcorrectionoflysosomalstorageinamucopolysaccharidosisIIIBmousemodelbyadeno-associatedvirus-mediatedgenedelivery.
MolTher2002;5:42–49.
7CressantA,DesmarisN,VerotL,BrejotT,FroissartR,VanierMTetal.
ImprovedbehaviorandneuropathologyinthemousemodelofSanlippotypeIIIBdiseaseafteradeno-associatedvirus-mediatedgenetransferinthestriatum.
JNeurosci2004;24:10229–10239.
8FuH,KangL,JenningsJS,MoySS,PerezA,DirosarioJetal.
SignicantlyincreasedlifespanandimprovedbehavioralperformancesbyrAAVgenedeliveryinadultmucopolysaccharidosisIIIBmice.
GeneTherapy2007;14:1065–1077.
9DiNataleP,DiDomenicoC,GargiuloN,CastaldoS,GonzalezYRE,MithbaokarPetal.
TreatmentofthemousemodelofmucopolysaccharidosistypeIIIBwithlentiviral-NAGLUvector.
BiochemJ2005;388:639–646.
10FuH,DiRosarioJ,KangL,MuenzerJ,McCartyDM.
Restorationofcentralnervoussystemalpha-N-acetylglucosaminidaseactivityandtherapeuticbenetsinmucopolysaccharidosisIIIBmicebyasingleintracisternalrecombinantadeno-associatedviraltype2vectordelivery.
JGeneMed2010;12:624–633.
11McCartyDM,DiRosarioJ,GulaidK,MuenzerJ,FuH.
Mannitol-facilitatedCNSentryofrAAV2vectorsignicantlydelayedtheneurologicaldiseaseprogressioninMPSIIIBmice.
GeneTherapy2009;16:1340–1352.
12HeldermonCD,OhlemillerKK,HerzogED,VoglerC,QinE,WozniakDFetal.
Therapeuticefcacyofbonemarrowtransplant,intracranialAAV-mediatedgenetherapy,orbothinthemousemodelofMPSIIIB.
MolTher2010;18:873–880.
13EllinwoodNM,AusseilJ,DesmarisN,BigouS,LiuS,JensJKetal.
Safe,efcient,andreproduciblegenetherapyofthebrainintheDogModelsofSanlippoandHurlerSyndromes.
MolTher2010;19:251–259.
14VoglerC,LevyB,GrubbJH,GalvinN,TanY,KakkisEetal.
Overcomingtheblood-brainbarrierwithhigh-doseenzymereplacementtherapyinmurinemucopoly-saccharidosisVII.
ProcNatlAcadSciUSA2005;102:14777–14782.
15SferraTJ,BackstromK,WangC,RennardR,MillerM,HuY.
Widespreadcorrectionoflysosomalstoragefollowingintrahepaticinjectionofarecombinantadeno-associatedvirusintheadultMPSVIImouse.
MolTher2004;10:478–491.
16LinD,DonsanteA,MacauleyS,LevyB,VoglerC,SandsMS.
Centralnervoussystem-directedAAV2/5-mediatedgenetherapysynergizeswithbonemarrowtransplantationinthemurinemodelofgloboid-cellleukodystrophy.
MolTher2007;15:44–52.
17DonsanteA,LevyB,VoglerC,SandsMS.
Clinicalresponsetopersistent,low-levelbeta-glucuronidaseexpressioninthemurinemodelofmucopolysaccharidosistypeVII.
JInheritMetabDis2007;30:227–238.
18SandsMS,BarkerJE.
Percutaneousintravenousinjectioninneonatalmice.
LabAnimSci1999;49:328–330.
19DeakinJA,LyonM.
Asimpliedandsensitiveuorescentmethodfordisaccharideanalysisofbothheparansulfateandchondroitin/dermatansulfatesfrombio-logicalsamples.
Glycobiology2008;18:483–491.
20LawrenceR,BrownJR,Al-MafrajiK,LamannaWC,BeitelJR,BoonsGJetal.
Disease-specicnon-reducingendcarbohydratebiomarkersformucopoly-saccharidoses.
NatChemBiol2012;8:197–204.
21LawrenceR,OlsonSK,SteeleRE,WangL,WarriorR,CummingsRDetal.
Evolutionarydifferencesinglycosaminoglycannestructuredetectedbyquan-titativeglycanreductiveisotopelabeling.
JBiolChem2008;283:33674–33684.
22YogalingamG,WeberB,MeehanJ,RogersJ,HopwoodJJ.
MucopolysaccharidosistypeIIIB:characterisationandexpressionofwild-typeandmutantrecombinantalpha-N-acetylglucosaminidaseandrelationshipwithsanlippophenotypeinanattenuatedpatient.
BiochimBiophysActa2000;1502:415–425.
23ColvilleGA,WattersJP,YuleW,BaxM.
SleepproblemsinchildrenwithSanlipposyndrome.
DevMedChildNeurol1996;38:538–544.
24GorlinR.
Genetichearinglossassociatedwithendocrineandmetabolicdisorders.
In:GorlinRJ,TorielloHV,CohenMM(eds).
HereditaryHearingLossanditsSyn-dromes.
OxfordMonographsonMedicalGeneticsvol.
28.
OxfordUniversityPress:NewYork,1995,pp318–354.
25BredenkampJK,SmithME,DudleyJP,WilliamsJC,CrumleyRL,CrockettDM.
Otolaryngologicmanifestationsofthemucopolysaccharidoses.
AnnOtolRhinolLaryngol1992;101:472–478.
26LeungLS,WeinsteinGW,HobsonRR.
Furtherelectroretinographicstudiesofpatientswithmucopolysaccharidoses.
BirthDefectsOrigArticSer1971;7:32–40.
27YoshimitsuM,SatoT,TaoK,WaliaJS,RasaiahVI,SleepGTetal.
BioluminescentimagingofamarkingtransgeneandcorrectionofFabrymicebyneonatalCorrectionofSanlippoSyndrometypeBCDHeldermonetal920GeneTherapy(2013)913–921&2013MacmillanPublishersLimitedinjectionofrecombinantlentiviralvectors.
ProcNatlAcadSciUSA2004;101:16909–16914.
28BifA,BartolomaeCC,CesanaD,CartierN,AubourgP,RanzaniMetal.
Lentiviralvectorcommonintegrationsitesinpreclinicalmodelsandaclinicaltrialreectabenignintegrationbiasandnotoncogenicselection.
Blood2011;117:5332–5339.
29WangGP,LevineBL,BinderGK,BerryCC,MalaniN,McGarrityGetal.
AnalysisoflentiviralvectorintegrationinHIVstudysubjectsreceivingautologousinfu-sionsofgenemodiedCD4Tcells.
MolTher2009;17:844–850.
30CartierN,Hacein-Bey-AbinaS,BartholomaeCC,VeresG,SchmidtM,KutscheraIetal.
HematopoieticstemcellgenetherapywithalentiviralvectorinX-linkedadrenoleukodystrophy.
Science2009;326:818–823.
31ZhengY,RyazantsevS,OhmiK,ZhaoHZ,RozengurtN,KohnDBetal.
RetrovirallytransducedbonemarrowhasatherapeuticeffectonbraininthemousemodelofmucopolysaccharidosisIIIB.
MolGenetMetab2004;82:286–295.
32FuH,DirosarioJ,KilledarS,ZaraspeK,McCartyDM.
CorrectionofneurologicaldiseaseofmucopolysaccharidosisIIIBinadultmicebyrAAV9trans-blood-brainbarriergenedelivery.
MolTher2011;19:1025–1033.
33MossRB,MillaC,ColomboJ,AccursoF,ZeitlinPL,ClancyJPetal.
RepeatedaerosolizedAAV-CFTRfortreatmentofcysticbrosis:arandomizedplacebo-controlledphase2Btrial.
HumGeneTher2007;18:726–732.
34HasbrouckNC,HighKA.
AAV-mediatedgenetransferforthetreatmentofhemophiliaB:problemsandprospects.
GeneTherapy2008;15:870–875.
35NathwaniAC,RosalesC,McIntoshJ,RastegarlariG,NathwaniD,RajDetal.
Long-termsafetyandefcacyfollowingsystemicadministrationofaself-complementaryAAVvectorencodinghumanFIXpseudotypedwithserotype5and8capsidproteins.
MolTher2011;19:876–885.
36LiH,MalaniN,HamiltonSR,SchlachtermanA,BussadoriG,EdmonsonSEetal.
AssessingthepotentialforAAVvectorgenotoxicityinamurinemodel.
Blood2010;117:3311–3319.
37ReissJ,HahnewaldR.
Molybdenumcofactordeciency:mutationsinGPHN,MOCS1,andMOCS2.
HumMutat2010;32:10–18.
38BellP,MoscioniAD,McCarterRJ,WuD,GaoG,HoangAetal.
AnalysisoftumorsarisinginmaleB6C3F1micewithandwithoutAAVvectordeliverytoliver.
MolTher2006;14:34–44.
39EmburyJE,FrostS,CharronCE,MadrigalE,PereraO,PoirierAEetal.
HepatitisvirusproteinX-phenylalaninehydroxylasefusionproteinsidentiedinPKUmicetreatedwithAAV-WPREvectors.
GeneTherapyMolBiol2008;12:69–76.
40DonsanteA,MillerDG,LiY,VoglerC,BruntEM,RussellDWetal.
AAVvectorintegrationsitesinmousehepatocellularcarcinoma.
Science2007;317:477.
41DonsanteA,VoglerC,MuzyczkaN,CrawfordJM,BarkerJ,FlotteTetal.
Observedincidenceoftumorigenesisinlong-termrodentstudiesofrAAVvectors.
GeneTherapy2001;8:1343–1346.
42RosasLE,GrievesJL,ZaraspeK,LaPerleKM,FuH,McCartyDM.
PatternsofscAAVvectorinsertionassociatedwithoncogeniceventsinamousemodelforgeno-toxicity.
MolTher2012;20:2098–2110.
43Hawkins-SalsburyJA,ReddyAS,SandsMS.
Combinationtherapiesforlysosomalstoragedisease:isthewholegreaterthanthesumofitspartsHumMolGenet2011;20:R54–R60.
44BiswasS,LeVineSM.
Substrate-reductiontherapyenhancesthebenetsofbonemarrowtransplantationinyoungmicewithgloboidcellleukodystrophy.
PediatrRes2002;51:40–47.
45BifA,CapotondoA,FasanoS,delCarroU,MarchesiniS,AzumaHetal.
Genetherapyofmetachromaticleukodystrophyreversesneurologicaldamageanddecitsinmice.
JClinInv2006;116:3070–3082.
46JeyakumarM,NorusF,TifftCJ,Cortina-BorjaM,ButtersTD,ProiaRLetal.
EnhancedsurvivalinSandhoffdiseasemicereceivingacombinationofsubstratedeprivationtherapyandbonemarrowtransplantation.
Blood2001;97:327–329.
47LeeJP,JeyakumarM,GonzalezR,TakahashiH,LeePJ,BaekRCetal.
Stemcellsactthroughmultiplemechanismstobenetmicewithneurodegenerativemetabolicdisease.
NatMed2007;13:439–447.
48MarshJ,FensomAH.
4-Methylumbelliferylalpha-N-acetylglucosaminidaseactivityfordiagnosisofSanlippoBdisease.
ClinGenet1985;27:258–262.
49SandsMS,BarkerJE,VoglerC,LevyB,GwynnB,GalvinNetal.
TreatmentofmurinemucopolysaccharidosistypeVIIbysyngeneicbonemarrowtransplanta-tioninneonates.
LabInvest1993;68:676–686.
50HerzogED,AtonSJ,NumanoR,SakakiY,TeiH.
Temporalprecisioninthemammaliancircadiansystem:areliableclockfromlessreliableneurons.
JBiolRhythms2004;19:35–46.
CorrectionofSanlippoSyndrometypeBCDHeldermonetal921&2013MacmillanPublishersLimitedGeneTherapy(2013)913–921
Wepreviouslyshowedthatintracranialadeno-associatedvirus(AAV)-basedgenetherapyresultsinpartialimprovementsofseveralaspectsofthedisease.
Inanattempttofurthercorrectthedisease,MPSIIIBmiceweretreatedat2–4daysofagewithintracranialAAV2/5-NAGLU(IC-AAV),intravenouslentiviral-NAGLU(IV-LENTI)orthecombinationofboth(BOTH).
TheBOTHgrouphadthemostcompletebiochemicalandhistologicalimprovementsofanytreatmentgroup.
ComparedwithuntreatedMPSIIIBanimals,alltreatmentsresultedinsignicantimprovementsinmotorfunction(rotarod)andhearing(auditory-evokedbrainstemresponse).
Inaddition,eachtreatmentgrouphadasignicantlyincreasedmedianlifespancomparedwiththeuntreatedgroup(322days).
Thecombinationarmhadthegreatestincrease(612days),followedbyIC-AAV(463days)andIV-LENTI(358days).
Finally,theBOTHgrouphadnearlynormalcircadianrhythmmeasureswithimprovementintimetoactivityonset.
Insummary,targetingboththesystemicandcentralnervoussystemdiseaseofMPSIIIBearlyinlifeappearstobethemostefcaciousapproachforthisinheritedmetabolicdisorder.
GeneTherapy(2013)20,913–921;doi:10.
1038/gt.
2013.
14;publishedonline28March2013Keywords:Sanlippo;behavioral;MPSIIIBINTRODUCTIONN-Acetyl-glucosaminidasedeciency(SanlippoSyndrometypeB,MucopolysaccharidosisIIIB)typicallycausesapediatriconsetdiseasecharacterizedphenotypically,byprogressivemotorandcognitivedeterioration,andhistologicallybyaccumulationoflysosomalinclusionsinmosttissues.
1Nocurrenttreatmentisapprovedinhumans.
Afterthegenewasidentied,2,3amurinemodelofmucopolysaccharidosistypeIIIB(MPSIIIB)wascreated.
4TheMPSIIIBmousesharesmanyofthebiochemical,histologicalandclinicalfeatureswiththehumandisease.
4,5SeveralgroupshavedemonstratedtheabilityofeitherintracranialorsystemicgenetherapyapproachestoreducelysosomaldistentioninthebrainsofMPSIIIBmice.
6–11Ourgroupalsodemonstratedimprovementsinhistologywithcorrespondingimprovementsinneurologicfunctionandlifespanusingintracranialgenetherapy.
12Intracranialdeliveryhasthusfardemonstratedthemostconsistentimprovementindiseaseprogression.
Increasesofapproximately30%inlifespanhavebeenobservedwithcentralnervoussystem(CNS)-directedtherapies.
AnintracranialgenetherapyapproachisnowbeingpursuedinalargeranimalmodelofMPSIIIB.
13Systemic-targetedgenetherapywasshowntoreducelysosomalstorageinperipheralorgans.
However,noneofthesesingleapproachescompletelyeradicatesintra-cytoplasmicinclusionsornormalizesthediseasephenotype.
WepreviouslyattemptedacombinationofCNS-directedgenetherapyandbonemarrowtransplantationwithlittleornobenetseenforthebonemarrowtransplantationarms.
However,thelevelofchimerismwasrelativelylowandtoxicitiesfromtheradiationconditioningwereevidentinthetransplantarm.
Inotherlysosomalstoragediseasemodels,therapiestotheCNSandtheperiphery,withenzymereplacement,bonemarrowtransplantationorgenetherapy,haveshowngreatlyimproveddiseasecorrectionespeciallywheninitiatedintheneo-natalperiod.
14–17Therefore,wehypothesizedthatneonatalcombinationtherapydirectedtoboththeCNSandtheperipherywouldprovidebettercorrectionofthedisease,especiallyifhighersystemiclevelsofN-acetylglucosaminidase(NAGLU)activitycouldbeattained.
Wedescribeherethebenetsobtainedfromeachmodeofgenetherapyandthesynergisticeffectofcombiningintracranialadeno-associatedvirus(AAV)-NAGLUandsystemiclentiviral-NAGLUgenetherapy.
RESULTSTreatmentsAllgenetherapyinjectionswereperformedinmicepupsat2–4daysofage.
IntracranialAAV-NAGLUtreatmentwas1DepartmentofMedicine,UniversityofFlorida,Gainesville,FL,USA;2DepartmentofInternalMedicine,WashingtonUniversity,StLouis,MO,USA;3DepartmentofOtorhinolaryngology,WashingtonUniversity,StLouis,MO,USA;4DepartmentofBiology,WashingtonUniversity,StLouis,MO,USA;5ZacharonPharmaceuticals,SanDiego,CA,USA;6DepartmentofPathology,SaintLouisUniversity,StLouis,MO,USA;7DepartmentofBiostatistics,UniversityofFlorida,Gainesville,FL,USAand8DepartmentofZoology,UniversityofWisconsin-Madison,Madison,WI,USA.
Correspondence:DrCDHeldermon,DepartmentofMedicine,UniversityofFlorida,1600SWArcherRoad,Box100278,Gainesville,FL32610,USA.
E-mail:coy.
heldermon@medicine.
u.
eduOrProfessorMSSands,DepartmentofInternalMedicine,WashingtonUniversityinStLouis,660SouthEuclid,CampusBox8007,StLouis,MO63110,USA.
E-mail:msands@dom.
wustl.
eduReceived14September2011;revised11February2013;accepted21February2013;publishedonline28March2013GeneTherapy(2013)20,913–921&2013MacmillanPublishersLimitedAllrightsreserved0969-7128/13www.
nature.
com/gtperformedasdescribedpreviouslywithsixdirectinjectionsof2mleachintofrontal,temporalandcerebellarregionsofthebrainofvectorataconcentrationof1.
51012viralparticlesperml.
5,12Intravenouslentiviral-NAGLUinjectionswerealsoperformedasdescribedpreviouslybyinjectionof100mlof1.
6108infectiousunitspermlviralaliquotintothesupercialtemporalvein.
18NAGLUactivityBiochemicalanalysisofN-acetyl-glucosaminidaseactivityforvariousorganswasdeterminedinmicefromeachgroupatB8monthsofageandcomparedwithuntreatedMPSIIIBanimals(MPSIIIBNOTX;Figure1).
TheMPSIIIBanimalsreceivingonlyintravenouslentiviralvectortreatment(MPSIIIBIV-LENTI)haddetectableNAGLUactivityinallorgansassayed(o2%ofnormalinthebrain(Po0.
05)andkidneys(notsignicant(NS)),11%inheart(Po0.
01),12%inlungs(NS),34%inliver(Po0.
001),34%inspleen(Po0.
05)and28%intheserum(NS;datanotshown).
Conversely,animalstreatedonlywithintracranialAAV(MPSIIIBIC-AAV)hadapproximately200%(Po0.
05),3%(NS)and5%(NS)ofnormalactivityinthebrain,liverandserum(datanotshown),respectively,andlittleornoactivityinthespleen,heart,lungsorkidneys.
Combinationtherapy(MPSIIIBBOTH)yieldedNAGLUactivitylevelsof424%forbrain(Po0.
01),13.
6%forliver(Po0.
001),6.
7%forheart(Po0.
01),19.
8%forspleen(Po0.
01),2.
9%forlungs(Po0.
05),42%forserum(NS)ando1%inthekidneys(NS).
Normalmicetreatedwithboththerapies(NORMALBOTH)hadnosignicantchangeinNAGLUactivityfromnormalmiceforthevisceralorgans(P40.
05foreverycomparison).
SecondaryenzymeactivityInmostlysosomalstoragedisorders,otherlysosomalenzymes,suchasb-glucuronidase(GUSB),areelevated.
TheresolutionofFigure1.
NAGLUactivitywasmeasuredinbrain,liver,heart,spleen,kidneysandlungsfromuntreatedandtreatedMPSIIIBmice.
Meanactivitylevelss.
e.
m.
foreachgrouprelativetotheNormalnotreatment(NOTX)grouparedepictedinthegraphs.
BOTH,bothIV-LENTIandIC-AAVtreatments;IC-AAV,intracranialadeno-associatedviralNAGLUvector;IV-LENTI,intravenouslentiviralNAGLUvector.
*Po0.
05;**Po0.
01;***Po0.
001comparedwithMPSIIIBNOTXgroup.
CorrectionofSanlippoSyndrometypeBCDHeldermonetal914GeneTherapy(2013)913–921&2013MacmillanPublishersLimitedthissecondaryelevationhasbeenusedasasurrogatefortherapeuticresponse.
SecondaryelevationsofGUSBactivityinthevariousgroupsaredepictedinFigure2andstatisticallycomparedwiththeMPSIIIBNOTXgroup.
UntreatedMPSIIIB(MPSIIIBNOTX)animalshadsignicantelevationsinGUSBcomparedwithnormalmiceforallorgansassayed:brain(358%,Po0.
001),liver(175%,Po0.
01),spleen(343%,Po0.
05),heart(619%,Po0.
05),lungs(645%,Po0.
001)andkidneys(439%,Po0.
001).
IC-AAVmicehadsignicantreductions(nearlynormal)insecondaryelevationscomparedwithuntreatedMPSIIIBmiceinthebrain(Po0.
0001),387%normalinthelungs(Po0.
01),butnosignicantreductionsintheothervisceralorgans.
IV-LENTI-treatedmicehadsignicantreductionsinGUSBactivityinthebrain(228%,Po0.
01),kidneys(217%,Po0.
05),andlungs(227%,Po0.
0001).
Combinationtreatmentresultedinnormalizationoflevelsinthebrain(95%,Po0.
0001),andsignicantreductionsinthekidneys(188%,Po0.
01)andlungs(183%,Po0.
0001).
GUSBactivityinthespleenandheartwerereducedinthecombinationarmbutdidnotreachsignicance(P0.
052and0.
099,respectively).
Thecombination-treatedwild-typeanimalsremainedwithinthenormalrangeexceptforthebrain,withasignicantreductionto82%ofnormal.
HistologyMPSIIIBBOTHmicehadthebestresponseintheCNS,withmarkedreductioninlysosomalstorageinglialandmeningealcellsaswellasincorticalneuronsasdepictedinFigure3.
ThesesamecelltypesinMPSIIIBIC-AAVmicehadalessstrikingbutdeniteresponsetotheintracranialtherapy.
MPSIIIBIV-LENTImicehadnoresponseinneuronsandonlymildreductioninstorageinglialandmeningealcells(Table1).
ThespleenandliverintreatedmiceFigure2.
Secondaryelevationofb-glucuronidaseactivityinbrain,liver,heart,spleen,kidneysandlungsfromuntreatedandtreatedMPSIIIBmice.
Meanactivitylevelss.
e.
m.
foreachgrouprelativetotheNormalNOTXgrouparedepictedinthegraphs.
GroupdesignationsareidenticaltothoseinFigure1(*Po0.
05,**Po0.
01,***Po0.
001,****Po0.
0001comparedwithMPSIIIBNOTXgroup).
CorrectionofSanlippoSyndrometypeBCDHeldermonetal915&2013MacmillanPublishersLimitedGeneTherapy(2013)913–921showedminimalornoresponsewithIC-AAVtherapyonly(Table1).
However,therewasaclearandsimilarreductioninstorageinthesesitesinboththeIV-LENTIandBOTHgroups.
Asagroup,themicetreatedwithcombinedIC-AAVandIV-LENTItherapyhadthebestresponsewiththegreatestreductioninstorage.
Glycosaminoglycan(GAG)analysisInordertoassessthelysosomalstoragemorequantitatively,theSensi-ProNon-ReducingEnd(NRE)assaywasperformedonbrainhomogenatesofadultmicefromeachgroupinordertomeasurethepathologicGAG(pGAG)accumulationofheparansulfate.
19–21Figure4depictsthereductioninbrainheparansulfatebyeachtreatment.
ComparedwiththeMPSIIIBNOTXgroup,MPSIIIBIV-LENTIhadnosignicantreductioninpGAG(85%ofNOTX,PNS).
MPSIIIBIC-AAV(24%ofNOTX,Po0.
01)andMPSIIIBBOTH(18%ofNOTX,Po0.
01)hadsignicantlyreducedpGAG.
NormalmicehadnodetectablepGAG.
CircadianactivityLong-termrunningwheelrecordingsfrom14to24weeksofagewereperformedwithmicefromeachexperimentalgroupundera12-hlight:12-hdarkcycle.
WepreviouslydescribedadifferencebetweenuntreatedMPSIIIBandnormalanimalsintwocircadianmeasurements:percentageofactivityoccurringduringthelightphase(greaterinMPSIIIB)andthetimefromlightsofftoactivityTable1.
LysosomalstorageinbrainandviscerabylightmicroscopyinMPSIIIBmiceaftertreatmentwithintracranialAAV,intravenouslentiviralorcombinedgenetherapyTissueIC-AAV(n4)IV-Lenti(n4)BOTH(n3)LiverHepatocytesNCNC-kNC-kkKupffercellsNC-kk-kkk-kkSpleenSinusliningcellsNC-kkk-kkkkkkBrainCorticalneuronsNC-kkNCkk-kkkMeningesk-kkkkkkGliakk-kkkNC-kkkkAbbreviations:IC-AAV,intracranialadeno-associatedvirus/5-N-acetylglu-cosaminidase;IV-LENTI,intravenouslentiviral-N-acetylglucosaminidase;MPSIIIB,mucopolysaccharidosistypeIIIB;NC,nochangeinlysosomalstoragefromage-matcheduntreatedMPSIIIBmouse.
k,Slightreductioninstorage;kk,moderatereductioninstorage;kkk,markedreductioninstorage,allcomparedwithage-matcheduntreatedMPSIIIBmouse.
Figure3.
Lysosomalinclusionsinparietalcortex.
Representativesectionsofparietalcortexof(a)MPSIIIB,(b)MPSIIIBIV-LENTI,(c)MPSIIIBIC-AAV(d)MPSIIIBBOTHmiceareshown.
Blackarrowsindicateneuronallysosomalinclusionsandwhitearrowsindicatelysosomaldistensioninglialcells.
Figure4.
Heparansulfatelevelsinthebrainofuntreatedandtreatedmice.
Pathologicglycosaminoglycan(pGAG)levelsinbrainhomogenatess.
e.
m.
foreachgrouparedepictedinpgpermgofprotein(N4–8foreachgroup,NS—notsignicant,**Po0.
01,****Po0.
0001comparedwiththeMPSIIIBNOTXgroup).
CorrectionofSanlippoSyndrometypeBCDHeldermonetal916GeneTherapy(2013)913–921&2013MacmillanPublishersLimitedonset(shorterinMPSIIIB).
5Similardifferenceswereobservedinthecurrentstudy,however,percentactivityduringthelightphasedidnotreachstatisticalsignicance(gurenotshown)aftercorrectionformultiplecomparisonsbetweentheMPSIIIBNOTX(mean15.
14%)andtheNormalNOTXgroup(mean9.
60%;P0.
14).
AlthoughtheaverageactivityoftheMPSIIIBIC-AAV(mean14.
23%)andMPSIIIBBOTH(mean12.
03%)groupswereincreasinglynumericallyimprovedfromtheMPSIIIBNOTXgroupandclosertotheNormalNOTXgroup,theywerenotstatisticallysignicantlydifferentaftercorrectionformultiplegroups.
Thetimeofdailyactivityonset(phaseangleofentrainment)wassignicantlyearlierintheMPSIIIBNOTXanimalscomparedwithNormalNOTXmice(P0.
007;Figure5).
TherewasnodifferenceinthephaseangleofentrainmentbetweentheMPSIIIBNOTXandtheMPSIIIBIV-LENTIgroups.
However,theMPSIIIBIC-AAVtrendedtowardimprovement(P0.
078)andMPSIIIBBOTHwassignicantlyimprovedrelativetotheuntreatedMPSIIIBmice(P0.
003).
TheMPSIIIBBOTHgroupphaseangleisnotdifferentthantheNormalNOTX(P0.
710)UntreatedMPSIIIBandnormalanimalshadnodifferenceintotaldailyactivitylevelsimilartopublishedresults.
5NoothertreatmentgroupwasdifferentthannormalorMPSIIIBuntreatedmicefortotalactivity.
Thecombination-treatednormalanimalswerenodifferentthanthenormaluntreatedanimalsforanycircadianparametertested.
AuditoryfunctionAuditory-evokedbrainstemresponsethresholdswereperformedoneachgroupatapproximately8–8.
5monthsofage.
Similartoourpreviousndings,MPSIIIBNOTXmicehadsignicantlydiminishedhearingcomparedwithnormalanimals,reachingthemaximumsoundoutputleveloftheequipmentatseveralfrequencieswithoutgeneratingaresponse(Figure6).
EachoftheMPSIIIBtreatmentgroupshadaconsistentlylowerresponsethresholdatallfrequenciestestedcomparedwithMPSIIIBNOTXmice.
Basedonagroup-wisecomparisonofthetreatmentgroups,thereappearedtobeatrendtowardslowerthresholdsintheIC-AAVgroupcomparedwiththeIV-LENTIgroup.
MPSIIIBBOTHmiceweresignicantlyimprovedcomparedwitheitherIC-AAVorIV-LENTImicebutremainssignicantlydifferentthantheNormalNOTXgroup(Po0.
001).
Surprisingly,theNormalBOTHanimalsalsohaddiminishedhearingcomparedwithNormalNOTXanimals.
Thispresumablyreectssomeadverseeffectofvirusadministrationonhearing,buttheNormalBOTHanimalsstillhaddemonstrablybetterhearingthresholdsthanMPSIIIBmice.
MotorfunctionWepreviouslydemonstratedIC-AAVtreatmentcoulddelaytheprogressionofmotordecitsinMPSIIIBmiceasmeasuredusingarockingrotarod.
Inthecurrentstudy,wedemonstratethateachofthetreatmentinterventionssignicantlydelayedtheprogressionofmotordysfunctioncomparedwithMPSIIIBNOTXanimals(Figure7).
Themediantimetoalatencyofp60sontherodincreasedfromB40weeksinMPSIIIBNOTXanimalstoB78weeksinMPSIIIBBOTHmice.
MPSIIIBIC-AAVmicereachedthemedianlatencyatB62weeksandMPSIIIBIV-LENTImicereachedthatlatencyatB52weeks.
ThetimefortheMPSIIIBBOTHmicetoreachthemedianlatencywassignicantlygreaterthananyotherMPSIIIBgroup.
ThemedianhadnotbeenreachedforeitherNormalgroupat90weeksofageandisstatisticallylongerthantheMPSIIIBBOTHgroup(P0.
05).
Figure6.
HearingsensitivityindecibelsoftreatedanduntreatedMPSIIIBandNormalmice.
Auditory-evokedbrainstemresponsesrecordingwasperformedat5,10,20,28.
3and40kHzon9–10animalspergroupat8–9monthsofage.
Eachgroup'smeandecibelthresholdtodetecttheaudiblestimulis.
d.
isshown.
AlltreatmentgroupsareimprovedwhencomparedwithMPSIIIBNOTXgroupoverallfrequencies(Po0.
001byrepeatedmeasureANOVA).
Figure5.
Timeofactivityonset.
Meantimesofdayofactivityonsetareplotteds.
e.
m.
Lightoffsetat1900hoursisindicatedwithgreyshading.
NotethatuntreatedMPSIIIBmiceandMPSIIIBmicetreatedwithlentivirusstartedtheirdailyrunningabout15minbeforelightsoff,whereasMPSIIIBmicetreatedwithAAVorbothlentivirusandAAVstartedrunningabout30minafterlightsoff,similartonormal,untreatedmice(n6foreachgroup,**Po0.
01comparedwithMPSIIIBNOTXgroup).
Figure7.
RockingrotarodperformanceLog-Rankanalysis.
Alatencyof60sorlesswasusedasathresholdtorepresentmotordysfunctionintheLog-Rankanalysisthatisgraphed.
Allgroups(n6–10)aresignicantlylongerthanMPSIIIBNOTX.
MPSIIIBBOTHissignicantlylongerthanIC-AAV(Po0.
05)andIV-LENTI(Po0.
001).
ThereisnodifferencebetweenNormalNOTXandNormalBOTHgroupssothelatterisnotshownforgureclarity(**Po0.
01,***Po0.
001comparedwithMPSIIIBNOTXgroup).
CorrectionofSanlippoSyndrometypeBCDHeldermonetal917&2013MacmillanPublishersLimitedGeneTherapy(2013)913–921LifespanWeshowedpreviouslythatIC-AAVtreatmentprolongsthelifeofMPSIIIBanimalsby4100days.
WendasimilarincreaseinmedianlifespanofIC-AAVinthisstudy(Figure8).
AlltreatmentgroupsdemonstrateasignicantincreaseinmediansurvivalrelativetoMPSIIIBNOTX(322days).
MPSIIIBIV-LENTImediansurvivalincreasedto358days,MPSIIIBIC-AAVto452daysandMPSIIIBBOTHto612days.
TherearenoapparentdifferencesinsurvivalbetweentheNormalandNormalBOTHgroupsupto720daysofage(datanotshown),andmediansurvivalhasnotbeenreachedinthesegroups,andissignicantlylongerthananyMPSIIIBtreatmentgroup.
AdverseeventsWehadtheopportunitytoexamineatleastfourmicefromallgroupsatB280daysofageandtwoMPSIIIBIC-AAV,twoMPSIIIBBOTHandnineNormalBOTHmicefrom588–726daysofagefortumorformation.
Noneofthemiceexaminedhaddemonstrabletumorsongrossexamination.
DISCUSSIONThemostimportantmeasuresofclinicalbenetforanytherapyarequalityoflifeandquantityoflife.
Wehavechosentheneonataltimeframefortreatmentbecauselysosomalstoragediseasetreatmentbybonemarrowtransplantinhumanshasdemonstratedthattheearliestpossibletreatmentmustbepursuedtopreventirreversibleneurologicdamage.
Thisislikelytobethemostefcaciousinterventiontimeframeinhumansoncenewbornscreeningforthisdisorderbecomescommonplace.
Wehavedemonstratedthatneonataltreatmentwitheitheranintravenousinjectionofalentiviralvector,intracranialinjectionofanAAVvectororthecombinationofbothcansignicantlyimprovesurrogatesforqualityoflife(motorfunction,hearingandcircadianrhythm)andincreaselifespan.
Inallcases,thesystemicapproachwaslessefcaciousthantheintracranialapproach.
Thisisnotsurprisinggiventhepredominantlyneurologicmanifesta-tionsofMPSIIIB.
However,thehumandiseasehasmanifestationsthatarenotintheCNS,suchashepatomegaly,hernias,diarrheaandearinfectionswitheffectsonhearingthatmaybeacombinationofcentralandperipheralneurologiceffectsandearstructureabnormalities.
22–26Inaddition,someofthebenetintheIV-LENTIgroupmayberelatedtotheverysmallamountofactivityobservedinthebrainsoftheseanimals,presumablefromasmallamountoflentiviraltransductionacrosstheblood–brainbarrierashasbeenseenbyotherinvestigators.
27Thismaybesupportedbytherelativelysmalldecreaseinbrainheparansulfateinthisgroup,whichwasnotstatisticallysignicant.
WealsocannotruleoutthepossibilitythatgreaterbenetmightbeachievablefromthesystemicapproachifweobtainedhigherexpressionofNAGLUbyeitherusingahigherorrepeateddoseofvector.
Althoughwewereabletoattainsupra-normallevelsinthebrainwiththecombinationofsystemicandintracranialtreatments,thelevelsofNAGLUactivitywerelessthanhalfnormalinallotherorgansassayed.
Interestingly,inourstudy,thecombinationapproachconsistentlyyieldedlowerorganactivitythanthesystemicmonotherapyandhigherbrainactivitythantheCNS-directedmonotherapy.
Thismaybeduetodisruptionoftheblood–brainbarrierduringtheCNSinjectionsallowingsystemicvectortoshunttothebrainreducingtheotherorganviralexposure.
WeattemptedtodeterminetherelativecontributionoflentiviralandAAVtransductioninthebrain.
However,becauseofthepresenceofrepetitiveelements,identicalinsertsandpoorDNAqualityafterhomogenizationneitherthequantitativePCRnorinsituhybridizationapproachestoquantifyAAVversuslentivirusweresuccessful.
WecannotruleoutthepossibilitythatthecombinationapproachanimalsreceivedagreatervectorcopynumberthantheIC-AAVgroup,despitethesameinjectionvolumefromthesamelotofvirus,thatcouldexplainthedifferencesbetweenthegroups.
Weobservedthatsomeorgans,suchaslungs,heartandkidneysseemmoreresistanttotransductionashasbeenobservedbyothergenetherapyinvestigations,27butstillhavesubstantialreductionofsecondaryenzymeelevation.
Thisabrogationofsecondaryelevationmaybethroughlow-levelNAGLUenzymecrosscorrection,frommorehighlytransducedorganssuchastheliverorspleen.
However,thislow-levelcrosscorrectionisnotenoughtomarkedlyreducelysosomalinclusionshistologically.
Perhapsthelevelofcrosscorrectionneededtoreducesecondaryenzymeelevationsislowerthanwhatisneededtomarkedlyreducelysosomaldistention.
Thishasbeenobservedwiththeliver-directedgenetherapyforMPSVIIaswell.
17Theadverseeffectsonauditory-evokedbrainstemresponsethresholdsfollowingvirusadministrationintheNormalgroupmostlikelydidnotresultfromAAVasthisgroupwasnotadverselyaffectedinourpriorstudy,whichevaluatedICAAVgiveninthesamemanner.
WhethertheadverseeffectsareattributabletoadirecteffectoflentivirusorrathertoxiceffectsofexcessNAGLUtonormalhearingisnotyetknown.
However,thiscouldbeaddressedbythedeterminationoflong-termeffectsonhearingstructureandfunctionaftersystemiclentiviraltreatmentofNormalmicewitheitherNAGLUorareporterenzyme.
Asweoriginallyhypothesized,thecombinationapproachappearstohavethegreatestbenet.
AlthoughMPSIIIBisasimplemonogenicdisease,ithasacomplexbiochemical,histologicalandclinicalphenotype.
AsNAGLUisexpressedinvirtuallyeverycellofthebody,mosttissueshavesomelysosomalstorage.
Consequently,multipletissuesmustbetreatedtoprovidethemaximumbenet.
Interestingly,thecombinationtherapyappearstobeadditiveforsomemeasuresthataffectqualityoflifesuchashearingandmotorfunction,andsynergisticformeasuresofcircadianfunction(differenceintimetoactivityonsetof2minwithIV-LENTI,22minwithIC-AAVand38minwithBOTH)andoverallsurvival(differenceinmediansurvivalof36dayswithIV-LENTI,130dayswithIC-AAVand290dayswithBOTH).
Notsurprisingly,thecombinationtreatmentwasnotbestedbyeithersingletherapyinanyfunctionalassessment.
Unfortunately,despitelong-termfunctionalimprovementsandsubstantialbenetsinsurvivalofthecombinationapproach,theprogressionofdiseaseduringthelastseveralweeksoflifeforeachoftheFigure8.
Survival.
AlltreatedmicewereanalyzedformediansurvivalbyKaplan–Meieranalysisandplotsareshown.
AlltreatedgroupshadsignicantlyimprovedsurvivalcomparedwithMPSIIIBNOTX.
MPSIIIBBOTHissignicantlylongerthanIC-AAV(Po0.
05)andIV-LENTI(Po0.
001).
ThereisnodifferencebetweenNormalNOTXandNormalBOTHgroupssothelatterisnotshownforgureclarity(**Po0.
01,***Po0.
001comparedwithMPSIIIBNOTXgroup).
CorrectionofSanlippoSyndrometypeBCDHeldermonetal918GeneTherapy(2013)913–921&2013MacmillanPublishersLimitedgroupsremainedsimilarwithpoorcoatcare,urinaryretentionandlossofbalance.
Thecombinationtherapyapproachusingtwodifferentvectorsinthecurrentstudyisnottheonlydualapproachthatcanbeenvisioned.
Weselectedalentiviralvectorforthesystemicapproachforitsefcacyatproducingsustainedexpressionofaproteinproductandthelackofknownimmuneinactivationinhumantrials.
28–30AhighersustainedlevelofNAGLUproductionmayhavebeenobtainedwithlentiviraltransducedbonemarrowselectedforhighenzymelevelashasbeendemonstratedbyothers.
31Alternatively,asystemicAAV9vectorapproachmayyieldsimilarsystemicbenetswithasingleperipheralinjectionsite,32however,thisserotypehasnotyetbeenusedinhumans.
Incontrasttolentivirustrials,humansystemicAAVgenetherapyapproacheshavebeenlimitedbyimmuneresponsestothevirus-infectedcells.
ThesystemicAAVapproachneedsfurtherstudytoavoidimmuneinactivationthathasbeenseeninhumantrials.
33,34Inadditiontodeterminingtheefcacyofadualgenetherapyapproach,thesafetyofAAVandlentiviralvectorsrequiresadditionalstudy.
Severalrecentstudieshavedemonstratedthelong-termsafetyofsystemicdeliveryofAAVvectorsinbothrodentsandnon-humanprimates.
35,36However,anumberofstudieshavealsoshownanincreaseintumorigenesisinAAV-treatedanimals.
37–42Givenrecentndingsregardingrecombinantlentiviralintegrationeventsnearactivegenes,36itseemsprudenttomonitorfortumorformationinfuturepre-clinicalexperimentsandhumantrials.
Wehavenotobservedanyhepatocellularcarcinomaorothermalignanciesinthecurrentstudydespitelongfollow-up.
Finally,othercombinationapproachescouldalsoprovetobeefcacious.
43Severalstudieshaveshownthatcombininghematopoieticstemcell-mediatedtherapywithgenetherapyorsubstratereductiontherapygreatlyincreasestheefcacyinthemurinemodelofKrabbedisease16,44andmetachromaticleukodystrophy.
45Itwasalsoshownthattheadditionofsmall-moleculeanti-inammatoryorsubstratereductionagentsenhancedtheefcacyofbothneuronalstemcell-andhematopoieticstemcell-mediatedtransplantationinthemurinemodelofSandhoffdisease.
46,47Thecaveattothisapproachistherelativelyhighmorbidityandmortalityofstemcelltransplantmethodsinhumansfromtheconditioningregimens,infections,andforallogeneictransplantsfromgraft-versus-hostdisease.
Inconclusion,combinationneonatalintracranialandsystemicNAGLUgenetherapyprovidessignicantandclinicallymean-ingfultherapeuticbenetinamousemodelofMPSIIIB.
However,studyofadditionalinterventionsiswarrantedasthecurrentstrategyisnotcompletelycorrective.
MATERIALSANDMETHODSViralconstructsAAV-NAGLUwasconstructedaspreviouslydescribedwithahuNAGLUcDNA(giftofElizabethNeufeld),AAV2genome,CMVenhancer,chickenb-actinpromoter,SV40poly-Asignaland30untranslatedregionfromtherabbitb-globingene.
VectorwasproducedattheUniversityofFloridaVectorCorewithapseudotypeAAV5capsid,anddilutedto1.
51012viralparticlespermlwithlactatedRinger'ssolutionbeforefreezingat851C.
Lentiviral-NAGLUwasconstructedwiththesamehuNAGLUcDNAintotheMNDvectorplasmid(kindgiftfromDKohn)withadelta-gag,centralpolypurinetractandthemyeloproliferativesarcomavirusenhancer,negativecontrolregiondeleted,Dl587revprimer-bindingsite-substituted(MND)promoter,SV40poly-Atailanddeltau330longterminalrepeat.
VectorwasproducedintheSands'labwithafour-plasmidsystemin293Tcellsandconcentratedto1.
6108infectiousunitspermlbeforealiquotingandfreezingat851C.
MiceC57BL/6NAGLU-decientmice(kindgiftfromENeufeld)weremaintainedbystrictsiblingmatingbyMSSatWashingtonUniversitySchoolofMedicine.
GenotypingwasdoneontissueofnewbornmicebyenzymeassayorNAGLUexon6andneomycininsertioncassettePCR.
AllproceduresonanimalswereinaccordancewiththeGuidelinesofInstitutionalAnimalCareandUseCommitteeatWashingtonUniversityinStLouis.
TreatmentsAt2–4daysofageallmicewereallocatedtotreatmentgroups:untreatedMPSIIIB(MPSIIIBNOTX,n19,15males),MPSIIIBtreatedwithintracranialAAV-NAGLU(MPSIIIBIC-AAV,n19,10males),MPSIIIBtreatedwithintravenouslentiviralNAGLU(MPSIIIBIV-LENTI,n19,7males),MPSIIIBtreatedwithbothintracranialAAV-NAGLUandintravenouslentiviralNAGLU(MPSIIIBBOTH,n16,9males),normaluntreated(NormalNOTX,n15,10males)andnormalwithbothintracranialAAV-NAGLUandintravenouslentiviralNAGLU(NormalBOTH,n17,8males).
Allgenetherapyinjectionswereperformedinmicepupsat2–4daysofage.
IntracranialAAV-NAGLUtreatmentwasperformedasdescribedpreviouslywithsixdirectinjectionsof2mleachintofrontal,temporalandcerebellarregionsofthebrainusinga32-Gneedle.
5,12Intravenouslentiviral-NAGLUinjectionswerealsoperformedasdescribedpreviouslybyinjectionof100mlofviralaliquotintothesupercialtemporalvein.
18Whencombined,theAAVinjectionswereperformedrstandthesystemicinjectionswereperformedwithin5–60min.
HistologyandbiochemistryMicefromeachgroup(n3–8forMPSIIIBNOTXandMPSIIIBBOTH,andn4–8allothergroups)between242and259daysofagewerekilledbyCO2asphyxiation.
Liver,spleen,kidneys,heart,lungsandbrainwereharvested.
Partofeachorganwasimmersionxedin2%gluteraldehyde/4%paraformaldehydeinphosphate-bufferedsalineandpartwasashfrozeninliquidnitrogenandstoredat851Cuntilmechanicalhomogenizationin10mMTris(pH7.
5),150mMNaCl,1mMdithiothreitoland0.
2%TritonX-100.
FixedtissuewasembeddedinSpurr'sresinand1-mm-thicksectionswerestainedwithtoluidinebluebeforeblindedevaluationoflysosomalstorageandvacuolization.
CelldebriswaspelletedandsupernatantswerecollectedforenzymeassaysofNAGLUandGUSBactivity.
DuplicateNAGLUassayswereperformedusing20mlofsupernatantaddedto40mlof0.
2mM4-methylumbelliferone-N-acetyl-a-D-glucopyranoside(Sigma,StLouis,MO,USA),0.
1MNaC2H3O2,0.
5mgml1bovineserumalbuminandincubatedat371C.
48Reactionswerestoppedwith1mlof0.
2MNa2CO3,0.
32Mglycine.
Substratecleavagewasdeterminedatexcitation365nmandemission448nmusingaHitachiF-2000FlourescenceSpectrophotometerusingastandardcurveof0.
5–5nmml1.
Specicactivitywascorrectedforproteinconcentration.
DuplicateGUSBassayswereperformedsimilarlyusingthe4-methylumbelliferoneenzymeassaymethodpreviouslydescribed.
49Activitylevelswereanalyzedbyone-wayanalysisofvariance(ANOVA)forcomparisonoftreatmentstoNOTXgroupsafterconrmationofadifferencebetweenNORMALandMPSIIIBNOTXgroupsbyStudent'st-test.
Brown-ForsytheTestofequalvarianceswassatisedasnotsignicantfororgancomparisonsofbrain,liver,heart,lungs,spleenandkidneysofGUSB.
GAGanalysisHomogenizedbrainsamples(N4–8foreachgroup,allfrommiceolderthan200days)werecodedwithanumericalidentierandsentblindedtoZacharonPharmaceuticalsInc.
(SanDiego,CA,USA)forpGAGanalysisusingSensi-ProNREassay.
pGAGaretheGAGfragmentspresentduetothedeciencyofthespeciclysosomalenzyme.
TheSensi-ProNREassayisahighlyspecicandsensitiveassaythatuseshigh-performanceliquidchromatographytoquantitatethereductioninlysosomalGAGaccumula-tion,bylabelingandquantifyingtheNREsoftheseGAGfragments.
InMPSIIIB,thepGAGmarkersareuniqueNRE-derivedtrisaccharidesthatterminateinN-acetylglucosamineduetothelysosomaldeciencyinN-acetylglucosaminidaseasdescribedindetailbyLawrenceetal.
20,21TheGAGswereextractedandpuriedbydiethylaminoethanol(DEAE)chromatography,digestedwithheparinlyases,uorescentlylabeledandanalyzedaspreviouslydescribed.
19One-wayANOVAwasusedtocomparepicogramsofpGAGpermicrogramofproteinforeachtreatmentgrouptotheMPSIIIBNOTXgroup.
CircadianassessmentsSixmalemicefromeachgroupwerestudiedfrom14to24weeksofageaspreviouslydescribed.
5AllmicewerehousedindividuallyincageswithaCorrectionofSanlippoSyndrometypeBCDHeldermonetal919&2013MacmillanPublishersLimitedGeneTherapy(2013)913–921runningwheelwithinlight-tightventilatedchambersilluminatedbyuorescentbulbs(F30T12-SP41-RS,GeneralElectric,Faireld,CT,USA,3.
91017to6.
91018photonss1m2)atthebottomofthecages.
Wheelrunningactivitywasrecordedin1minbins(Clocklab,Actimetrics,Evanston,IL,USA),whereasmicewereexposedtoalight–darkschedule(lightsonat0700andoffat1900hours).
Weanalyzedphaseangleofentrainment(delaybetweendailylightoffsetandonsetofactivity),totaldailyactivityandproportionofdailyactivityinthelightphaseofthephotocycleusingClocklab.
50Statisticalanalysiswasbyrepeated-measuresANOVA.
AuditoryevaluationAuditory-evokedbrainstemresponseswereperformedat8–8.
5monthsofageusingproceduressimilartothosedescribedpreviously.
5,12Micewereanesthetizedwithketamine/xylazine(85/15mgkg1,i.
p.
)whilecoretemperaturewasmaintainedat37.
0±1.
01Cbyathermostaticallyregulatedheatingpadmonitoredviaarectalprobe(YellowSprings).
Platinumneedleelectrodes(Grass,WestWarwick,RI,USA)wereplacedsubcutaneouslyintheback,vertexandbehindtherightearwhileconnectingtoaGrassP15differentialamplier(100–10000Hz,100).
ACambridgeElectronicDesignMicro1401(CambridgeElectronicDesign,Cambridge,UK)runningSIGNALandcustomaveragingsoftwaredigitizedthesignalat30kHz.
Toneburststimuliat5,10,20,28.
3and40kHzweredelivered1000timesat20s1usinganAlpineSPS-OEOAcoaxialspeaker(Crutcheld,Charlottesville,VA,USA)located10cmlateraltotherightear.
Ateachtestfrequency,thestimuluslevelwasreducedin5dBminimumstepstodeterminetheminimumsoundpressurelevelrequiredforvisualdetectionofWaveI.
Repeated-measureANOVAwithDunnetsmultiplecomparisoncorrectionwasperformedtocompareeachtreatmentgrouptotheMPSIIIBNOTXcontrol.
Separateone-wayANOVAwasperformedfordatafromallgroupsateachtestfrequency,followedbyBonferronimultiplecomparisontests.
Samplesizesbygroupwereasfollows:MPSIIIBNOTX,n10;MPSIIIBIC-AAV,n9;MPSIIIBIV-LENTI,n9;MPSIIIBBOTH,n10;NormalNOTX,n10;NormalBOTH,n10.
MotorfunctionassessmentAswedescribedpreviouslyinthismodel5,12miceweretrainedonarotarod(UGOBasile,Varese,Italy)movingataspeedof10r.
p.
m.
thatswitcheddirectionaftereachfullrotationforupto180sperattempt,withthreeattemptsperday.
Testsweredoneevery28daysfrom196upto672daysofage(MPSIIIBNOTX,n6;MPSIIIBIC-AAV,n9;MPSIIIBIV-LENTI,n10;MPSIIIBBOTH,n10;NormalNOTX,n9;NormalBOTH,n8).
Longestlatencytofallfromtherotarodofthethreeattemptswasusedforcomparisons.
DatawereinterpretedusingaLogRanktestfortimetolatencyofo60sonrotarod.
LifespanAlltreatedanimalswereanalyzedbyintentiontotreatwithaGehan–Wilcoxontest.
Kaplan–Meiercurvesweregeneratedtoassesstheeffectoftreatmentsonsurvival.
CONFLICTOFINTERESTJillianRBrownandBrettECrawfordarefull-timeemployeesandshareholdersofZacharonPharmaceuticals.
Allotherauthorshavenoconictofinteresttoreport.
ACKNOWLEDGEMENTSThisworkwasfundedinpartbyNIHgrantsNS043205(MSS),HD055461(MSS),K08DK085141-01(CDH)andbytheSanlippoChildren'sResearchFoundation(CDH).
REFERENCES1YogalingamG,HopwoodJJ.
MoleculargeneticsofmucopolysaccharidosistypeIIIAandIIIB:diagnostic,clinical,andbiologicalimplications.
HumMutat2001;18:264–281.
2WeberB,BlanchL,ClementsPR,ScottHS,HopwoodJJ.
CloningandexpressionofthegeneinvolvedinSanlippoBsyndrome(mucopolysaccharidosisIIIB).
HumMolGenet1996;5:771–777.
3ZhaoHG,LiHH,BachG,SchmidtchenA,NeufeldEF.
ThemolecularbasisofSanlipposyndrometypeB.
ProcNatlAcadSciUSA1996;93:6101–6105.
4LiHH,YuWH,RozengurtN,ZhaoHZ,LyonsKM,AnagnostarasSetal.
MousemodelofSanlipposyndrometypeBproducedbytargeteddisruptionofthegeneencodingalpha-N-acetylglucosaminidase.
ProcNatlAcadSciUSA1999;96:14505–14510.
5HeldermonCD,HennigAK,OhlemillerKK,OgilvieJM,HerzogED,BreidenbachAetal.
Developmentofsensory,motorandbehavioraldecitsinthemurinemodelofSanlipposyndrometypeB.
PLoSOne2007;2:e772.
6FuH,SamulskiRJ,McCownTJ,PicornellYJ,FletcherD,MuenzerJ.
NeurologicalcorrectionoflysosomalstorageinamucopolysaccharidosisIIIBmousemodelbyadeno-associatedvirus-mediatedgenedelivery.
MolTher2002;5:42–49.
7CressantA,DesmarisN,VerotL,BrejotT,FroissartR,VanierMTetal.
ImprovedbehaviorandneuropathologyinthemousemodelofSanlippotypeIIIBdiseaseafteradeno-associatedvirus-mediatedgenetransferinthestriatum.
JNeurosci2004;24:10229–10239.
8FuH,KangL,JenningsJS,MoySS,PerezA,DirosarioJetal.
SignicantlyincreasedlifespanandimprovedbehavioralperformancesbyrAAVgenedeliveryinadultmucopolysaccharidosisIIIBmice.
GeneTherapy2007;14:1065–1077.
9DiNataleP,DiDomenicoC,GargiuloN,CastaldoS,GonzalezYRE,MithbaokarPetal.
TreatmentofthemousemodelofmucopolysaccharidosistypeIIIBwithlentiviral-NAGLUvector.
BiochemJ2005;388:639–646.
10FuH,DiRosarioJ,KangL,MuenzerJ,McCartyDM.
Restorationofcentralnervoussystemalpha-N-acetylglucosaminidaseactivityandtherapeuticbenetsinmucopolysaccharidosisIIIBmicebyasingleintracisternalrecombinantadeno-associatedviraltype2vectordelivery.
JGeneMed2010;12:624–633.
11McCartyDM,DiRosarioJ,GulaidK,MuenzerJ,FuH.
Mannitol-facilitatedCNSentryofrAAV2vectorsignicantlydelayedtheneurologicaldiseaseprogressioninMPSIIIBmice.
GeneTherapy2009;16:1340–1352.
12HeldermonCD,OhlemillerKK,HerzogED,VoglerC,QinE,WozniakDFetal.
Therapeuticefcacyofbonemarrowtransplant,intracranialAAV-mediatedgenetherapy,orbothinthemousemodelofMPSIIIB.
MolTher2010;18:873–880.
13EllinwoodNM,AusseilJ,DesmarisN,BigouS,LiuS,JensJKetal.
Safe,efcient,andreproduciblegenetherapyofthebrainintheDogModelsofSanlippoandHurlerSyndromes.
MolTher2010;19:251–259.
14VoglerC,LevyB,GrubbJH,GalvinN,TanY,KakkisEetal.
Overcomingtheblood-brainbarrierwithhigh-doseenzymereplacementtherapyinmurinemucopoly-saccharidosisVII.
ProcNatlAcadSciUSA2005;102:14777–14782.
15SferraTJ,BackstromK,WangC,RennardR,MillerM,HuY.
Widespreadcorrectionoflysosomalstoragefollowingintrahepaticinjectionofarecombinantadeno-associatedvirusintheadultMPSVIImouse.
MolTher2004;10:478–491.
16LinD,DonsanteA,MacauleyS,LevyB,VoglerC,SandsMS.
Centralnervoussystem-directedAAV2/5-mediatedgenetherapysynergizeswithbonemarrowtransplantationinthemurinemodelofgloboid-cellleukodystrophy.
MolTher2007;15:44–52.
17DonsanteA,LevyB,VoglerC,SandsMS.
Clinicalresponsetopersistent,low-levelbeta-glucuronidaseexpressioninthemurinemodelofmucopolysaccharidosistypeVII.
JInheritMetabDis2007;30:227–238.
18SandsMS,BarkerJE.
Percutaneousintravenousinjectioninneonatalmice.
LabAnimSci1999;49:328–330.
19DeakinJA,LyonM.
Asimpliedandsensitiveuorescentmethodfordisaccharideanalysisofbothheparansulfateandchondroitin/dermatansulfatesfrombio-logicalsamples.
Glycobiology2008;18:483–491.
20LawrenceR,BrownJR,Al-MafrajiK,LamannaWC,BeitelJR,BoonsGJetal.
Disease-specicnon-reducingendcarbohydratebiomarkersformucopoly-saccharidoses.
NatChemBiol2012;8:197–204.
21LawrenceR,OlsonSK,SteeleRE,WangL,WarriorR,CummingsRDetal.
Evolutionarydifferencesinglycosaminoglycannestructuredetectedbyquan-titativeglycanreductiveisotopelabeling.
JBiolChem2008;283:33674–33684.
22YogalingamG,WeberB,MeehanJ,RogersJ,HopwoodJJ.
MucopolysaccharidosistypeIIIB:characterisationandexpressionofwild-typeandmutantrecombinantalpha-N-acetylglucosaminidaseandrelationshipwithsanlippophenotypeinanattenuatedpatient.
BiochimBiophysActa2000;1502:415–425.
23ColvilleGA,WattersJP,YuleW,BaxM.
SleepproblemsinchildrenwithSanlipposyndrome.
DevMedChildNeurol1996;38:538–544.
24GorlinR.
Genetichearinglossassociatedwithendocrineandmetabolicdisorders.
In:GorlinRJ,TorielloHV,CohenMM(eds).
HereditaryHearingLossanditsSyn-dromes.
OxfordMonographsonMedicalGeneticsvol.
28.
OxfordUniversityPress:NewYork,1995,pp318–354.
25BredenkampJK,SmithME,DudleyJP,WilliamsJC,CrumleyRL,CrockettDM.
Otolaryngologicmanifestationsofthemucopolysaccharidoses.
AnnOtolRhinolLaryngol1992;101:472–478.
26LeungLS,WeinsteinGW,HobsonRR.
Furtherelectroretinographicstudiesofpatientswithmucopolysaccharidoses.
BirthDefectsOrigArticSer1971;7:32–40.
27YoshimitsuM,SatoT,TaoK,WaliaJS,RasaiahVI,SleepGTetal.
BioluminescentimagingofamarkingtransgeneandcorrectionofFabrymicebyneonatalCorrectionofSanlippoSyndrometypeBCDHeldermonetal920GeneTherapy(2013)913–921&2013MacmillanPublishersLimitedinjectionofrecombinantlentiviralvectors.
ProcNatlAcadSciUSA2004;101:16909–16914.
28BifA,BartolomaeCC,CesanaD,CartierN,AubourgP,RanzaniMetal.
Lentiviralvectorcommonintegrationsitesinpreclinicalmodelsandaclinicaltrialreectabenignintegrationbiasandnotoncogenicselection.
Blood2011;117:5332–5339.
29WangGP,LevineBL,BinderGK,BerryCC,MalaniN,McGarrityGetal.
AnalysisoflentiviralvectorintegrationinHIVstudysubjectsreceivingautologousinfu-sionsofgenemodiedCD4Tcells.
MolTher2009;17:844–850.
30CartierN,Hacein-Bey-AbinaS,BartholomaeCC,VeresG,SchmidtM,KutscheraIetal.
HematopoieticstemcellgenetherapywithalentiviralvectorinX-linkedadrenoleukodystrophy.
Science2009;326:818–823.
31ZhengY,RyazantsevS,OhmiK,ZhaoHZ,RozengurtN,KohnDBetal.
RetrovirallytransducedbonemarrowhasatherapeuticeffectonbraininthemousemodelofmucopolysaccharidosisIIIB.
MolGenetMetab2004;82:286–295.
32FuH,DirosarioJ,KilledarS,ZaraspeK,McCartyDM.
CorrectionofneurologicaldiseaseofmucopolysaccharidosisIIIBinadultmicebyrAAV9trans-blood-brainbarriergenedelivery.
MolTher2011;19:1025–1033.
33MossRB,MillaC,ColomboJ,AccursoF,ZeitlinPL,ClancyJPetal.
RepeatedaerosolizedAAV-CFTRfortreatmentofcysticbrosis:arandomizedplacebo-controlledphase2Btrial.
HumGeneTher2007;18:726–732.
34HasbrouckNC,HighKA.
AAV-mediatedgenetransferforthetreatmentofhemophiliaB:problemsandprospects.
GeneTherapy2008;15:870–875.
35NathwaniAC,RosalesC,McIntoshJ,RastegarlariG,NathwaniD,RajDetal.
Long-termsafetyandefcacyfollowingsystemicadministrationofaself-complementaryAAVvectorencodinghumanFIXpseudotypedwithserotype5and8capsidproteins.
MolTher2011;19:876–885.
36LiH,MalaniN,HamiltonSR,SchlachtermanA,BussadoriG,EdmonsonSEetal.
AssessingthepotentialforAAVvectorgenotoxicityinamurinemodel.
Blood2010;117:3311–3319.
37ReissJ,HahnewaldR.
Molybdenumcofactordeciency:mutationsinGPHN,MOCS1,andMOCS2.
HumMutat2010;32:10–18.
38BellP,MoscioniAD,McCarterRJ,WuD,GaoG,HoangAetal.
AnalysisoftumorsarisinginmaleB6C3F1micewithandwithoutAAVvectordeliverytoliver.
MolTher2006;14:34–44.
39EmburyJE,FrostS,CharronCE,MadrigalE,PereraO,PoirierAEetal.
HepatitisvirusproteinX-phenylalaninehydroxylasefusionproteinsidentiedinPKUmicetreatedwithAAV-WPREvectors.
GeneTherapyMolBiol2008;12:69–76.
40DonsanteA,MillerDG,LiY,VoglerC,BruntEM,RussellDWetal.
AAVvectorintegrationsitesinmousehepatocellularcarcinoma.
Science2007;317:477.
41DonsanteA,VoglerC,MuzyczkaN,CrawfordJM,BarkerJ,FlotteTetal.
Observedincidenceoftumorigenesisinlong-termrodentstudiesofrAAVvectors.
GeneTherapy2001;8:1343–1346.
42RosasLE,GrievesJL,ZaraspeK,LaPerleKM,FuH,McCartyDM.
PatternsofscAAVvectorinsertionassociatedwithoncogeniceventsinamousemodelforgeno-toxicity.
MolTher2012;20:2098–2110.
43Hawkins-SalsburyJA,ReddyAS,SandsMS.
Combinationtherapiesforlysosomalstoragedisease:isthewholegreaterthanthesumofitspartsHumMolGenet2011;20:R54–R60.
44BiswasS,LeVineSM.
Substrate-reductiontherapyenhancesthebenetsofbonemarrowtransplantationinyoungmicewithgloboidcellleukodystrophy.
PediatrRes2002;51:40–47.
45BifA,CapotondoA,FasanoS,delCarroU,MarchesiniS,AzumaHetal.
Genetherapyofmetachromaticleukodystrophyreversesneurologicaldamageanddecitsinmice.
JClinInv2006;116:3070–3082.
46JeyakumarM,NorusF,TifftCJ,Cortina-BorjaM,ButtersTD,ProiaRLetal.
EnhancedsurvivalinSandhoffdiseasemicereceivingacombinationofsubstratedeprivationtherapyandbonemarrowtransplantation.
Blood2001;97:327–329.
47LeeJP,JeyakumarM,GonzalezR,TakahashiH,LeePJ,BaekRCetal.
Stemcellsactthroughmultiplemechanismstobenetmicewithneurodegenerativemetabolicdisease.
NatMed2007;13:439–447.
48MarshJ,FensomAH.
4-Methylumbelliferylalpha-N-acetylglucosaminidaseactivityfordiagnosisofSanlippoBdisease.
ClinGenet1985;27:258–262.
49SandsMS,BarkerJE,VoglerC,LevyB,GwynnB,GalvinNetal.
TreatmentofmurinemucopolysaccharidosistypeVIIbysyngeneicbonemarrowtransplanta-tioninneonates.
LabInvest1993;68:676–686.
50HerzogED,AtonSJ,NumanoR,SakakiY,TeiH.
Temporalprecisioninthemammaliancircadiansystem:areliableclockfromlessreliableneurons.
JBiolRhythms2004;19:35–46.
CorrectionofSanlippoSyndrometypeBCDHeldermonetal921&2013MacmillanPublishersLimitedGeneTherapy(2013)913–921
- 7.76aav.com相关文档
- 881.9976aav.com
- Interactive76aav.com
- A46676aav.com
- applications76aav.com
- capsid76aav.com
- July76aav.com
ParkInHost - 俄罗斯VPS主机 抗投诉 55折,月付2.75欧元起
ParkInHost主机商是首次介绍到的主机商,这个商家是2013年的印度主机商,隶属于印度DiggDigital公司,主营业务有俄罗斯、荷兰、德国等机房的抗投诉虚拟主机、VPS主机和独立服务器。也看到商家的数据中心还有中国香港和美国、法国等,不过香港机房肯定不是直连的。根据曾经对于抗投诉外贸主机的了解,虽然ParkInHost以无视DMCA的抗投诉VPS和抗投诉服务器,但是,我们还是要做好数据备...

Megalayer新加坡服务器国际带宽线路测评
前几天有关注到Megalayer云服务器提供商有打算在月底的时候新增新加坡机房,这个是继美国、中国香港、菲律宾之外的第四个机房。也有工单询问到官方,新加坡机房有包括CN2国内优化线路和国际带宽,CN2优化线路应该是和菲律宾差不多的。如果我们追求速度和稳定性的中文业务,建议还是选择CN2优化带宽的香港服务器。这里有要到Megalayer新加坡服务器国际带宽的测试服务器,E3-1230配置20M国际带...
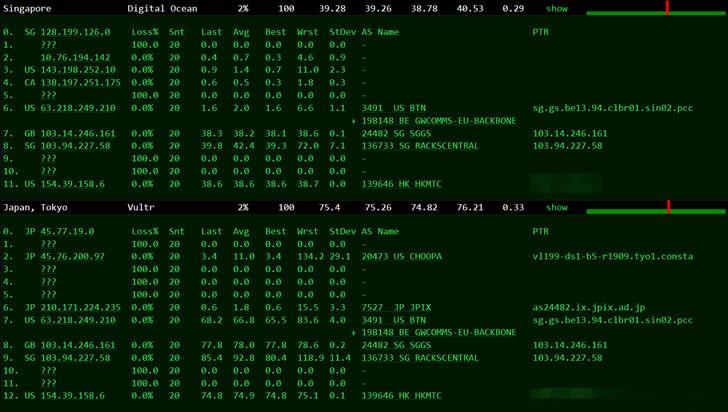
TabbyCloud周年庆&七夕节活动 美國INAP 香港CN2
TabbyCloud迎来一周岁的生日啦!在这一年里,感谢您包容我们的不足和缺点,在您的理解与建议下我们也在不断改变与成长。为庆祝TabbyCloud运营一周年和七夕节,TabbyCloud推出以下活动。TabbyCloud周年庆&七夕节活动官方网站:https://tabbycloud.com/香港CN2: https://tabbycloud.com/cart.php?gid=16购买链...
76aav.com为你推荐
-
www.kk4kk.com猪猪影院www.mlzz.com 最新电影收费吗?m.kan84.net电视剧海派甜心全集海派甜心在线观看海派甜心全集高清dvd快播迅雷下载bbs2.99nets.com天堂1单机版到底怎么做dadi.tv1223tv影院首页地址是什么?1223tv影院在哪里可以找到?m.yushuwu.org花样滑冰名将YU NA KIM的资料谁有?www.jsjtxx.com苏州考驾照,理论考试结束后,要在网上学习满12小时,网站是什么雀嘴鳝长嘴鳄(雀鳝)如何分雌雄恋战千年千年修炼千年妖,打一个生肖!carlymilo卡莱米路(Carlymilo)的品牌介绍ip查寻自己的IP怎么查?