Servicesrewrite
rewrite 时间:2021-01-25 阅读:()
U.
S.
Food&DrugAdministration10903NewHampshireAvenueDocID#04017.
03.
08SilverSpring,MD20993www.
fda.
govMarch29,2019SpryHealth,Inc.
℅CraigCoombsConsultantCoombsMedicalDeviceConsulting,Inc.
1193ShermanSt.
Alameda,CA94501Re:K181352Trade/DeviceName:LoopSystemRegulationNumber:21CFR870.
2700RegulationName:OximeterRegulatoryClass:ClassIIProductCode:DQA,BZQDated:March4,2019Received:March5,2019DearCraigCoombs:WehavereviewedyourSection510(k)premarketnotificationofintenttomarketthedevicereferencedaboveandhavedeterminedthedeviceissubstantiallyequivalent(fortheindicationsforusestatedintheenclosure)tolegallymarketedpredicatedevicesmarketedininterstatecommercepriortoMay28,1976,theenactmentdateoftheMedicalDeviceAmendments,ortodevicesthathavebeenreclassifiedinaccordancewiththeprovisionsoftheFederalFood,Drug,andCosmeticAct(Act)thatdonotrequireapprovalofapremarketapprovalapplication(PMA).
Youmay,therefore,marketthedevice,subjecttothegeneralcontrolsprovisionsoftheAct.
Althoughthisletterreferstoyourproductasadevice,pleasebeawarethatsomeclearedproductsmayinsteadbecombinationproducts.
The510(k)PremarketNotificationDatabaselocatedathttps://www.
accessdata.
fda.
gov/scripts/cdrh/cfdocs/cfpmn/pmn.
cfmidentifiescombinationproductsubmissions.
ThegeneralcontrolsprovisionsoftheActincluderequirementsforannualregistration,listingofdevices,goodmanufacturingpractice,labeling,andprohibitionsagainstmisbrandingandadulteration.
Pleasenote:CDRHdoesnotevaluateinformationrelatedtocontractliabilitywarranties.
Weremindyou,however,thatdevicelabelingmustbetruthfulandnotmisleading.
Ifyourdeviceisclassified(seeabove)intoeitherclassII(SpecialControls)orclassIII(PMA),itmaybesubjecttoadditionalcontrols.
ExistingmajorregulationsaffectingyourdevicecanbefoundintheCodeofFederalRegulations,Title21,Parts800to898.
Inaddition,FDAmaypublishfurtherannouncementsconcerningyourdeviceintheFederalRegister.
K181352-CraigCoombsPage2PleasebeadvisedthatFDA'sissuanceofasubstantialequivalencedeterminationdoesnotmeanthatFDAhasmadeadeterminationthatyourdevicecomplieswithotherrequirementsoftheActoranyFederalstatutesandregulationsadministeredbyotherFederalagencies.
YoumustcomplywithalltheAct'srequirements,including,butnotlimitedto:registrationandlisting(21CFRPart807);labeling(21CFRPart801);medicaldevicereporting(reportingofmedicaldevice-relatedadverseevents)(21CFR803)fordevicesorpostmarketingsafetyreporting(21CFR4,SubpartB)forcombinationproducts(seehttps://www.
fda.
gov/CombinationProducts/GuidanceRegulatoryInformation/ucm597488.
htm);goodmanufacturingpracticerequirementsassetforthinthequalitysystems(QS)regulation(21CFRPart820)fordevicesorcurrentgoodmanufacturingpractices(21CFR4,SubpartA)forcombinationproducts;and,ifapplicable,theelectronicproductradiationcontrolprovisions(Sections531-542oftheAct);21CFR1000-1050.
Also,pleasenotetheregulationentitled,"Misbrandingbyreferencetopremarketnotification"(21CFRPart807.
97).
ForquestionsregardingthereportingofadverseeventsundertheMDRregulation(21CFRPart803),pleasegotohttp://www.
fda.
gov/MedicalDevices/Safety/ReportaProblem/default.
htm.
Forcomprehensiveregulatoryinformationaboutmedicaldevicesandradiation-emittingproducts,includinginformationaboutlabelingregulations,pleaseseeDeviceAdvice(https://www.
fda.
gov/MedicalDevices/DeviceRegulationandGuidance/)andCDRHLearn(http://www.
fda.
gov/Training/CDRHLearn).
Additionally,youmaycontacttheDivisionofIndustryandConsumerEducation(DICE)toaskaquestionaboutaspecificregulatorytopic.
SeetheDICEwebsite(http://www.
fda.
gov/DICE)formoreinformationorcontactDICEbyemail(DICE@fda.
hhs.
gov)orphone(1-800-638-2041or301-796-7100).
Sincerely,BramD.
Zuckerman,M.
D.
DirectorDivisionofCardiovascularDevicesOfficeofDeviceEvaluationCenterforDevicesandRadiologicalHealthEnclosureShawnW.
Forrest-S2019.
03.
2910:28:03-04'00'FORMFDA3881(7/17)Page1of1PSCPublishingServices(301)443-6740EFDEPARTMENTOFHEALTHANDHUMANSERVICESFoodandDrugAdministrationIndicationsforUseFormApproved:OMBNo.
0910-0120ExpirationDate:06/30/2020SeePRAStatementbelow.
510(k)Number(ifknown)K181352DeviceNameLoopSystemIndicationsforUse(Describe)TheLoopSystemisintendedforadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofphysiologicalparametersthatwilllaterbetransmittedtoawebserverforremotereviewbyaclinician.
TheLoopSystemmeasuresandrecords:arterialoxygensaturation(SpO2)heartrate(HR)respirationrate(RR)AllofthesemeasurementsaremadewhennomotionisdetectedbytheSystem.
TheLoopSystemdevicedoesnotprovidephysiologicalalarmsTypeofUse(Selectoneorboth,asapplicable)PrescriptionUse(Part21CFR801SubpartD)Over-The-CounterUse(21CFR801SubpartC)CONTINUEONASEPARATEPAGEIFNEEDED.
ThissectionappliesonlytorequirementsofthePaperworkReductionActof1995.
*DONOTSENDYOURCOMPLETEDFORMTOTHEPRASTAFFEMAILADDRESSBELOW.
*Theburdentimeforthiscollectionofinformationisestimatedtoaverage79hoursperresponse,includingthetimetoreviewinstructions,searchexistingdatasources,gatherandmaintainthedataneededandcompleteandreviewthecollectionofinformation.
Sendcommentsregardingthisburdenestimateoranyotheraspectofthisinformationcollection,includingsuggestionsforreducingthisburden,to:DepartmentofHealthandHumanServicesFoodandDrugAdministrationOfficeofChiefInformationOfficerPaperworkReductionAct(PRA)StaffPRAStaff@fda.
hhs.
gov"Anagencymaynotconductorsponsor,andapersonisnotrequiredtorespondto,acollectionofinformationunlessitdisplaysacurrentlyvalidOMBnumber.
"K181352:LoopSystemTraditionalPremarketNotificationPage1Section5:510(k)SummaryA.
DeviceInformationCategoryCommentsSponsor:SpryHealth,Inc235AlmaStPaloAlto,CA94301Tel:(650)352-3429PrimaryCommunicantCraigCoombsCoombsMedicalDeviceConsulting1193ShermanStreetAlameda,CA94501Tel:510-337-0140DeviceCommonName:OximeterBreathingFrequencyMonitorDeviceClassificationNumber:21CFR870.
270021CFR868.
2375DeviceClassification&ProductCode:Class2,DQA&BZQDeviceProprietaryName:LoopSystemPredicateDeviceInformation:PredicateDevice:MightySatRXFingertipPulseOximeterPredicateDeviceManufacturer:MasimoPredicateDeviceCommonName:OximeterBreathingFrequencyMonitorPredicateDevicePremarketNotification#K181956PredicateDeviceClassification:21CFR870.
2700:Oximeter21CFR868.
2375:BreathingFrequencyMonitorPredicateDeviceClass&ProductCode:Class2,DQA&BZQB.
DateSummaryPrepared13March2019C.
DescriptionofDeviceTheLoopSystemisaprescription-onlymedicaldeviceindicatedforusebyadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofresting(i.
e.
,nomotion)physiologicalparameters.
Thedataisderivedfromreflectance-basedphotoplethysmogram(PPG)signalsfromthepatient'swrist,collectedbyLightEmittingDiodesK181352:LoopSystemTraditionalPremarketNotificationPage2(LEDs)ofvaryingwavelengthsandphotodiodessensitivetosaidwavelengthsembeddedintheLoopBand.
AnaccelerometerincorporatedintheLoopBanddetermineswhenthepatientisatrestbyconstantlymonitoringtheactivitylevelofthepatient.
Thedataisrecordedduringthoserestingperiods.
Usingfilteringtechnologyforremovalofnoise(includingambientlight)anddataprocessingalgorithms,theLoopSystemstorestherawdatacollecteduntilitisabletosendtotheSpryServer.
PatientsweartheLoopBandontheirwristforperiodsofupto24hours.
PatientswillthenhavetochargetheLoopBandwithaLoopChargingStationprovidedtothemaspartoftheLoopSystem.
Duringcharging,thedataisuploadedtotheSpryServer.
TheLoopbandcanindependentlyanalyzeanddisplayrestingheartrateandarterialoxygensaturation(SpO2).
ThedatamustbedownloadedandanalyzedbytheSpryServertodeterminerespirationrate.
AllphysiologicalmeasurementscollectedbytheLoopSystemarereportedonacommaseparatedvalue(CSV)fileasatime-stampedseries.
TheCSVcanthenberemotelyaccessedthroughawebinterfaceforreviewandanalysisbyaclinician.
D.
IndicationsforUseTheLoopSystemisintendedforadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofphysiologicalparametersthatwilllaterbetransmittedtoawebserverforremotereviewbyaclinician.
TheLoopSystemmeasuresandrecords:arterialoxygensaturation(SpO2)heartrate(HR)respirationrate(RR)AllofthesemeasurementsaremadewhennomotionisdetectedbytheSystem.
TheLoopSystemdevicedoesnotprovidephysiologicalalarmsK181352:LoopSystemTraditionalPremarketNotificationPage3E.
ComparisontoPredicateDeviceTheapplicationSpryHealthLoopSystemissubstantiallyequivalenttotheMasimoMightySatRxFingertipPulseOximeter(K181956).
ThedeviceshaveasimilarIndicationsforUse,features,technologyandaccuracy.
PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceIntendedUseIssuesIndicationsforUseTheMasimoMightySatRxFingertipPulseOximeterisintendedforhospitals,hospital-typefacilities,homeenvironments,andtransport.
TheMasimoMightySatRxFingertipPulseOximeterisindicatedforthenoninvasivespotcheckingoffunctionaloxygensaturationofarterialhemoglobin(SpO2)andpulserate(PR)foradultandpediatricpatientsduringbothnomotionandmotionconditions,andforpatientswhoarewellorpoorlyperfused.
TheMasimoMightySatRxFingertipPulseOximeterisindicatedforthenoninvasivespotcheckingofrespirationrate(RRp)foradultpatients.
TheLoopSystemisintendedforadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofphysiologicalparametersthatwilllaterbetransmittedtoawebserverforremotereviewbyaclinician.
TheLoopSystemmeasuresandrecords:arterialoxygensaturation(SpO2)heartrate(HR)respirationrate(RR)AllofthesemeasurementsaremadewhennomotionisdetectedbytheSystem.
TheLoopSystemdevicedoesnotprovidephysiologicalalarms.
TheapplicationLoopSystemIndicationsforUseincludesasubsetofthepatientsanduseconditionsclearedinthepredicatedevice.
TheLoopSystemandthepredicateareintendedforadultsinahomeenvironmentwhenthewearersarenotmoving.
Sincetheapplicationdeviceisintendedforasubsetoftheapplicationconditionsofthepredicateddevice,nonewtypesofsafetyorefficacyquestionsareraised.
BothdevicesprovideSpO2,HR,andRR.
Neitherdeviceisintendedforcontinuousvitalsignmonitoring.
ProductCodeRegulationDescriptionDQA-OximeterBZQ–BreathingFrequencyMonitorDQA–OximeterBZQ–BreathingFrequencyMonitorArterialOxygensaturationandderivedHRandRRcapabilitiesallfallunderthesameproductcodesforbothdevices.
PatientPopulationHR&SpO2-Adults,pediatricsRespiratoryRate–AdultsNon-criticalcareAdultpatientsforallparametersNon-criticalcareTheapplicationdeviceintendedcohortisasubsetofthepredicate's;nonewtypesofsafetyorefficacyquestionsareraised.
K181352:LoopSystemTraditionalPremarketNotificationPage4PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceUseEnvironmenthospitals,hospital-typefacilities,homeenvironments,andtransport.
homeenvironmentTheapplicationdeviceuseenvironmentisasubsetofthepredicate's;nonewtypesofsafetyorefficacyquestionsareraised.
PrescribedPrescriptionuseonlyPrescriptionuseonlyIdenticalParameterSamplingFrequencySpotcheckparametersasdesiredbyusers.
Notdesignedforcontinuouswearing.
DesignedfornearlycontinuousmeasuringandrecordingofphysiologicaldatawhenthepatientisnotinmotionDifferenceinsamplingfrequencydoesnotraisenewsafetyorefficacyquestionsTechnologyassociatedwitheachparameterRespirationRateFundamentalScientificTechnologyRespirationrate(RR)measuredbyanalyzingcyclicvariationsinthephotoplethysmogram.
Thediodesaremountedinthedevicesuchthattheyareincontactwiththeskin.
SameTechnologicallyequivalentRespirationRateRange4–70respirationsperminute(RPM)4-40RPMApplicationdevicehasalowerupperlimitthanpredicate.
SpecificRPMsthatare>40arenotclinicallydifferentiatinginaretrospectivemonitoringcontextRespirationRateAccuracy3RPMARMS3RPMARMSAclinicalstudydemonstratedthattheLoopSystemcouldaccuratelymonitorrespirationrateaswellasthepredicate,withinthesensorrangeArterialhemoglobinoxygensaturation(SpO2)FundamentalScientificTechnologySpO2measuredbyanalyzingreflectanceofcertainLEDfrequenciesinaphotoplethysmogramdesign.
Thediodesaremountedinthedevicesuchthattheyareincontactwiththeskin.
SameTechnologicallyequivalentK181352:LoopSystemTraditionalPremarketNotificationPage5PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceSpO2Range70–100%70–100%IdenticalSpO2Accuracy2%ARMS,nomotion3%ARMS,nomotionBothcomplywithISO80601-2-61aswellaswithFDAGuidanceforPulseOximeters(2013)HeartRate(HR)FundamentalScientificTechnologyHRmeasuredbyanalyzingcyclicvariationsinreflectanceofcertainLEDfrequenciesinaphotoplethysmogramdesign.
Thediodesaremountedinthedevicesuchthattheyareincontactwiththeskin.
SameTechnologicallyequivalentHeartRateRange25–240beatsperminute(BPM)25–250beatsperminute(BPM)Clinicallyequivalent.
BothcomplywithISO80601-2-61HeartRateAccuracy3BPMARMS,nomotion3BPMARMS,nomotionClinicallyequivalent.
BothcomplywithISO80601-2-61StandardsComplianceIEC60601-1IEC60601-1-2IEC60601-1-11ISO80601-2-61ISO10993-1IEC60601-1IEC60601-1-2IEC60601-1-11IEC60601-1-6ISO80601-2-61ISO10993-1BothdevicesmeetlatestguidelinesforsafetyandefficacyDesignUserInterfaceFingerclampWristbandBothdevicesareworntoprovidedeviceaccesstoperipheralarteries.
Differenceinparticularuserinterfacedoesnotraisenewquestionsofsafetyorefficacy.
K181352:LoopSystemTraditionalPremarketNotificationPage6PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceWearinglocationandfrequencyForintermittentwearing,aka"spotchecking"Continuouswearing,nearlycontinuousrecordingofalldata.
Differenceinwearinglocationonthebodyandfrequencydoesnotraisenewquestionsofsafetyorefficacy.
Bothlocationsallowformonitoringperipheralarteries.
DisplayOLEDcolordisplayofHRandSpO2whendevicewornLEDcolordisplayofHRandSpO2ondemandSimilarPowersource2AAbatteriesInternalrechargeablebatteriesBothbattery-poweredDataCommunicationWireless(Bluetoothpairing)withmobiledevicessuchassmartphonesWireless(cellularconnection)viachargingstationtoSpryServer.
Bothdevicesaredesignedtotransmittheirdatatoalternatedevicesorsites.
TypeofprotectionUnknownTypeBF–AppliedPartperIEC60601-1ApplicationdevicemeetslatestguidelinesforelectricalsafetyPatientcontractingmaterialsPlasticPlasticSimilarBiocompatibilityComplianttoISO10993-1ComplianttoISO10993-1IdenticalK181352:LoopSystemTraditionalPremarketNotificationPage7F.
SummaryofSupportingDataSpryHealthhasconductedextensivetestingtoensurethattheLoopSystemmetdesignspecifications,functionsasintended,andconformstointernationallyrecognizedstandardsandFDAGuidelines.
BenchTestingAlltestresultsdemonstratetheperformanceofSpryHealthLoopSystemmettherequirementsofitspre-definedacceptancecriteriaandintendeduse.
Theresultsofthebench(non-clinical)testingdemonstratethattheLoopSystemisassafeandeffectiveasthepredicatedevice.
BenchtestingdemonstratedtheaccuracyoftheheartratemonitoringwiththeLoopSystem.
ThistestingwasconductedinaccordancewithISO80601-2-61Medicalelectricalequipment-Part2-61:ParticularrequirementsforbasicsafetyandessentialperformanceofpulseoximeterequipmentandinaccordancewiththeFDAGuidelinesforPulseOximeters-PremarketNotificationSubmissions[510(k)s]:GuidanceforIndustryandFoodandDrugAdministrationStaff(2013).
TheLoopSystemwasfoundtobeincompliancewithbothdocuments.
Additionally,transittestingandone-yearshelflifetestingwasconducted.
ElectricalSafetyandElectromagneticCompatibilityTestingElectricalsafetytestingwasconductedinaccordancewith:IEC60601-1:2005+AM1:2012Medicalelectricalequipment-Part1:GeneralrequirementsforbasicsafetyandessentialperformanceIEC60601-1-2:2014:MedicalElectricalEquipment–Part1-2:GeneralRequirementsforSafety–Collateralstandard:ElectromagneticDisturbances–RequirementsandtestsIEC60601-1-11:2015Medicalelectricalequipment–Part1-11:Generalrequirementsforbasicsafetyandessentialperformance–CollateralStandard:RequirementsformedicalelectricalequipmentandmedicalelectricalsystemsusedinthehomehealthcareenvironmentIEC60601-1-6:2013Medicalelectricalequipment–Part1-6:Generalrequirementsforbasicsafetyandessentialperformance–Collateralstandard:UsabilityTheSpryHealthLoopSystempassedallelectricalsafetyandEMCtesting.
BiocompatibilityTestingBiocompatibilitytestingwasconductedinaccordancewithISO10993-1:2009/AC:2010,Biologicalevaluationofmedicaldevices–Part1:Evaluationandtestingwithinariskmanagementprocess,andFDA'sguidancedocuments,UseofInternationalStandardISO10993-1,"Biologicalevaluationofmedicaldevices-Part1:Evaluationandtestingwithinariskmanagementprocess"issuedJune16,2016.
ThistestingdemonstratesthatthematerialsintheLoopBandwillnotcauseanadversebiocompatibilityreactionwhenusedasintended.
K181352:LoopSystemTraditionalPremarketNotificationPage8ClinicalStudiesTwoclinicalstudieswereconductedtodemonstratetheperformanceoftheLoopSystem.
Oneclinicalstudyinvestigatedtheaccuracyofrespirationratemonitoringin12healthyadultsubjectswitharangeofskintypesandrespiratoryrates.
TheLoopSystemaccuracywasshowntobeequivalenttoagoldstandardmonitoringprocedure.
Anotherclinicalstudyinvestigatedtheaccuracyofthepulseoximetrymonitoringin12healthyadultsubjectswitharangeofskintypes.
TheLoopSystemaccuracywascomparedtothegoldstandard,arterialbloodgasanalysis.
ThistestingwasconductedinaccordancewithISO80601-2-61Medicalelectricalequipment-Part2-61:ParticularrequirementsforbasicsafetyandessentialperformanceofpulseoximeterequipmentandinaccordancewiththeFDAGuidelinesforPulseOximeters-PremarketNotificationSubmissions[510(k)s]:GuidanceforIndustryandFoodandDrugAdministrationStaff(2013).
TheLoopSystemwasfoundtobeincompliancewithbothdocuments.
G.
ConclusionSpryHealthconcludesthattheapplicationSpryHealthLoopSystemissubstantiallyequivalenttotheMasimoMightySatRxFingertipPulseOximeter(K181956).
ThedeviceshaveasimilarIndicationsforUse,features,technologyandaccuracyinmonitoringrespirationrate,heartrateandpulseoximetrysaturation.
S.
Food&DrugAdministration10903NewHampshireAvenueDocID#04017.
03.
08SilverSpring,MD20993www.
fda.
govMarch29,2019SpryHealth,Inc.
℅CraigCoombsConsultantCoombsMedicalDeviceConsulting,Inc.
1193ShermanSt.
Alameda,CA94501Re:K181352Trade/DeviceName:LoopSystemRegulationNumber:21CFR870.
2700RegulationName:OximeterRegulatoryClass:ClassIIProductCode:DQA,BZQDated:March4,2019Received:March5,2019DearCraigCoombs:WehavereviewedyourSection510(k)premarketnotificationofintenttomarketthedevicereferencedaboveandhavedeterminedthedeviceissubstantiallyequivalent(fortheindicationsforusestatedintheenclosure)tolegallymarketedpredicatedevicesmarketedininterstatecommercepriortoMay28,1976,theenactmentdateoftheMedicalDeviceAmendments,ortodevicesthathavebeenreclassifiedinaccordancewiththeprovisionsoftheFederalFood,Drug,andCosmeticAct(Act)thatdonotrequireapprovalofapremarketapprovalapplication(PMA).
Youmay,therefore,marketthedevice,subjecttothegeneralcontrolsprovisionsoftheAct.
Althoughthisletterreferstoyourproductasadevice,pleasebeawarethatsomeclearedproductsmayinsteadbecombinationproducts.
The510(k)PremarketNotificationDatabaselocatedathttps://www.
accessdata.
fda.
gov/scripts/cdrh/cfdocs/cfpmn/pmn.
cfmidentifiescombinationproductsubmissions.
ThegeneralcontrolsprovisionsoftheActincluderequirementsforannualregistration,listingofdevices,goodmanufacturingpractice,labeling,andprohibitionsagainstmisbrandingandadulteration.
Pleasenote:CDRHdoesnotevaluateinformationrelatedtocontractliabilitywarranties.
Weremindyou,however,thatdevicelabelingmustbetruthfulandnotmisleading.
Ifyourdeviceisclassified(seeabove)intoeitherclassII(SpecialControls)orclassIII(PMA),itmaybesubjecttoadditionalcontrols.
ExistingmajorregulationsaffectingyourdevicecanbefoundintheCodeofFederalRegulations,Title21,Parts800to898.
Inaddition,FDAmaypublishfurtherannouncementsconcerningyourdeviceintheFederalRegister.
K181352-CraigCoombsPage2PleasebeadvisedthatFDA'sissuanceofasubstantialequivalencedeterminationdoesnotmeanthatFDAhasmadeadeterminationthatyourdevicecomplieswithotherrequirementsoftheActoranyFederalstatutesandregulationsadministeredbyotherFederalagencies.
YoumustcomplywithalltheAct'srequirements,including,butnotlimitedto:registrationandlisting(21CFRPart807);labeling(21CFRPart801);medicaldevicereporting(reportingofmedicaldevice-relatedadverseevents)(21CFR803)fordevicesorpostmarketingsafetyreporting(21CFR4,SubpartB)forcombinationproducts(seehttps://www.
fda.
gov/CombinationProducts/GuidanceRegulatoryInformation/ucm597488.
htm);goodmanufacturingpracticerequirementsassetforthinthequalitysystems(QS)regulation(21CFRPart820)fordevicesorcurrentgoodmanufacturingpractices(21CFR4,SubpartA)forcombinationproducts;and,ifapplicable,theelectronicproductradiationcontrolprovisions(Sections531-542oftheAct);21CFR1000-1050.
Also,pleasenotetheregulationentitled,"Misbrandingbyreferencetopremarketnotification"(21CFRPart807.
97).
ForquestionsregardingthereportingofadverseeventsundertheMDRregulation(21CFRPart803),pleasegotohttp://www.
fda.
gov/MedicalDevices/Safety/ReportaProblem/default.
htm.
Forcomprehensiveregulatoryinformationaboutmedicaldevicesandradiation-emittingproducts,includinginformationaboutlabelingregulations,pleaseseeDeviceAdvice(https://www.
fda.
gov/MedicalDevices/DeviceRegulationandGuidance/)andCDRHLearn(http://www.
fda.
gov/Training/CDRHLearn).
Additionally,youmaycontacttheDivisionofIndustryandConsumerEducation(DICE)toaskaquestionaboutaspecificregulatorytopic.
SeetheDICEwebsite(http://www.
fda.
gov/DICE)formoreinformationorcontactDICEbyemail(DICE@fda.
hhs.
gov)orphone(1-800-638-2041or301-796-7100).
Sincerely,BramD.
Zuckerman,M.
D.
DirectorDivisionofCardiovascularDevicesOfficeofDeviceEvaluationCenterforDevicesandRadiologicalHealthEnclosureShawnW.
Forrest-S2019.
03.
2910:28:03-04'00'FORMFDA3881(7/17)Page1of1PSCPublishingServices(301)443-6740EFDEPARTMENTOFHEALTHANDHUMANSERVICESFoodandDrugAdministrationIndicationsforUseFormApproved:OMBNo.
0910-0120ExpirationDate:06/30/2020SeePRAStatementbelow.
510(k)Number(ifknown)K181352DeviceNameLoopSystemIndicationsforUse(Describe)TheLoopSystemisintendedforadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofphysiologicalparametersthatwilllaterbetransmittedtoawebserverforremotereviewbyaclinician.
TheLoopSystemmeasuresandrecords:arterialoxygensaturation(SpO2)heartrate(HR)respirationrate(RR)AllofthesemeasurementsaremadewhennomotionisdetectedbytheSystem.
TheLoopSystemdevicedoesnotprovidephysiologicalalarmsTypeofUse(Selectoneorboth,asapplicable)PrescriptionUse(Part21CFR801SubpartD)Over-The-CounterUse(21CFR801SubpartC)CONTINUEONASEPARATEPAGEIFNEEDED.
ThissectionappliesonlytorequirementsofthePaperworkReductionActof1995.
*DONOTSENDYOURCOMPLETEDFORMTOTHEPRASTAFFEMAILADDRESSBELOW.
*Theburdentimeforthiscollectionofinformationisestimatedtoaverage79hoursperresponse,includingthetimetoreviewinstructions,searchexistingdatasources,gatherandmaintainthedataneededandcompleteandreviewthecollectionofinformation.
Sendcommentsregardingthisburdenestimateoranyotheraspectofthisinformationcollection,includingsuggestionsforreducingthisburden,to:DepartmentofHealthandHumanServicesFoodandDrugAdministrationOfficeofChiefInformationOfficerPaperworkReductionAct(PRA)StaffPRAStaff@fda.
hhs.
gov"Anagencymaynotconductorsponsor,andapersonisnotrequiredtorespondto,acollectionofinformationunlessitdisplaysacurrentlyvalidOMBnumber.
"K181352:LoopSystemTraditionalPremarketNotificationPage1Section5:510(k)SummaryA.
DeviceInformationCategoryCommentsSponsor:SpryHealth,Inc235AlmaStPaloAlto,CA94301Tel:(650)352-3429PrimaryCommunicantCraigCoombsCoombsMedicalDeviceConsulting1193ShermanStreetAlameda,CA94501Tel:510-337-0140DeviceCommonName:OximeterBreathingFrequencyMonitorDeviceClassificationNumber:21CFR870.
270021CFR868.
2375DeviceClassification&ProductCode:Class2,DQA&BZQDeviceProprietaryName:LoopSystemPredicateDeviceInformation:PredicateDevice:MightySatRXFingertipPulseOximeterPredicateDeviceManufacturer:MasimoPredicateDeviceCommonName:OximeterBreathingFrequencyMonitorPredicateDevicePremarketNotification#K181956PredicateDeviceClassification:21CFR870.
2700:Oximeter21CFR868.
2375:BreathingFrequencyMonitorPredicateDeviceClass&ProductCode:Class2,DQA&BZQB.
DateSummaryPrepared13March2019C.
DescriptionofDeviceTheLoopSystemisaprescription-onlymedicaldeviceindicatedforusebyadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofresting(i.
e.
,nomotion)physiologicalparameters.
Thedataisderivedfromreflectance-basedphotoplethysmogram(PPG)signalsfromthepatient'swrist,collectedbyLightEmittingDiodesK181352:LoopSystemTraditionalPremarketNotificationPage2(LEDs)ofvaryingwavelengthsandphotodiodessensitivetosaidwavelengthsembeddedintheLoopBand.
AnaccelerometerincorporatedintheLoopBanddetermineswhenthepatientisatrestbyconstantlymonitoringtheactivitylevelofthepatient.
Thedataisrecordedduringthoserestingperiods.
Usingfilteringtechnologyforremovalofnoise(includingambientlight)anddataprocessingalgorithms,theLoopSystemstorestherawdatacollecteduntilitisabletosendtotheSpryServer.
PatientsweartheLoopBandontheirwristforperiodsofupto24hours.
PatientswillthenhavetochargetheLoopBandwithaLoopChargingStationprovidedtothemaspartoftheLoopSystem.
Duringcharging,thedataisuploadedtotheSpryServer.
TheLoopbandcanindependentlyanalyzeanddisplayrestingheartrateandarterialoxygensaturation(SpO2).
ThedatamustbedownloadedandanalyzedbytheSpryServertodeterminerespirationrate.
AllphysiologicalmeasurementscollectedbytheLoopSystemarereportedonacommaseparatedvalue(CSV)fileasatime-stampedseries.
TheCSVcanthenberemotelyaccessedthroughawebinterfaceforreviewandanalysisbyaclinician.
D.
IndicationsforUseTheLoopSystemisintendedforadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofphysiologicalparametersthatwilllaterbetransmittedtoawebserverforremotereviewbyaclinician.
TheLoopSystemmeasuresandrecords:arterialoxygensaturation(SpO2)heartrate(HR)respirationrate(RR)AllofthesemeasurementsaremadewhennomotionisdetectedbytheSystem.
TheLoopSystemdevicedoesnotprovidephysiologicalalarmsK181352:LoopSystemTraditionalPremarketNotificationPage3E.
ComparisontoPredicateDeviceTheapplicationSpryHealthLoopSystemissubstantiallyequivalenttotheMasimoMightySatRxFingertipPulseOximeter(K181956).
ThedeviceshaveasimilarIndicationsforUse,features,technologyandaccuracy.
PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceIntendedUseIssuesIndicationsforUseTheMasimoMightySatRxFingertipPulseOximeterisintendedforhospitals,hospital-typefacilities,homeenvironments,andtransport.
TheMasimoMightySatRxFingertipPulseOximeterisindicatedforthenoninvasivespotcheckingoffunctionaloxygensaturationofarterialhemoglobin(SpO2)andpulserate(PR)foradultandpediatricpatientsduringbothnomotionandmotionconditions,andforpatientswhoarewellorpoorlyperfused.
TheMasimoMightySatRxFingertipPulseOximeterisindicatedforthenoninvasivespotcheckingofrespirationrate(RRp)foradultpatients.
TheLoopSystemisintendedforadultpatientsinthehomeenvironmentforpassive,non-invasive,intermittentdatacollectionofphysiologicalparametersthatwilllaterbetransmittedtoawebserverforremotereviewbyaclinician.
TheLoopSystemmeasuresandrecords:arterialoxygensaturation(SpO2)heartrate(HR)respirationrate(RR)AllofthesemeasurementsaremadewhennomotionisdetectedbytheSystem.
TheLoopSystemdevicedoesnotprovidephysiologicalalarms.
TheapplicationLoopSystemIndicationsforUseincludesasubsetofthepatientsanduseconditionsclearedinthepredicatedevice.
TheLoopSystemandthepredicateareintendedforadultsinahomeenvironmentwhenthewearersarenotmoving.
Sincetheapplicationdeviceisintendedforasubsetoftheapplicationconditionsofthepredicateddevice,nonewtypesofsafetyorefficacyquestionsareraised.
BothdevicesprovideSpO2,HR,andRR.
Neitherdeviceisintendedforcontinuousvitalsignmonitoring.
ProductCodeRegulationDescriptionDQA-OximeterBZQ–BreathingFrequencyMonitorDQA–OximeterBZQ–BreathingFrequencyMonitorArterialOxygensaturationandderivedHRandRRcapabilitiesallfallunderthesameproductcodesforbothdevices.
PatientPopulationHR&SpO2-Adults,pediatricsRespiratoryRate–AdultsNon-criticalcareAdultpatientsforallparametersNon-criticalcareTheapplicationdeviceintendedcohortisasubsetofthepredicate's;nonewtypesofsafetyorefficacyquestionsareraised.
K181352:LoopSystemTraditionalPremarketNotificationPage4PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceUseEnvironmenthospitals,hospital-typefacilities,homeenvironments,andtransport.
homeenvironmentTheapplicationdeviceuseenvironmentisasubsetofthepredicate's;nonewtypesofsafetyorefficacyquestionsareraised.
PrescribedPrescriptionuseonlyPrescriptionuseonlyIdenticalParameterSamplingFrequencySpotcheckparametersasdesiredbyusers.
Notdesignedforcontinuouswearing.
DesignedfornearlycontinuousmeasuringandrecordingofphysiologicaldatawhenthepatientisnotinmotionDifferenceinsamplingfrequencydoesnotraisenewsafetyorefficacyquestionsTechnologyassociatedwitheachparameterRespirationRateFundamentalScientificTechnologyRespirationrate(RR)measuredbyanalyzingcyclicvariationsinthephotoplethysmogram.
Thediodesaremountedinthedevicesuchthattheyareincontactwiththeskin.
SameTechnologicallyequivalentRespirationRateRange4–70respirationsperminute(RPM)4-40RPMApplicationdevicehasalowerupperlimitthanpredicate.
SpecificRPMsthatare>40arenotclinicallydifferentiatinginaretrospectivemonitoringcontextRespirationRateAccuracy3RPMARMS3RPMARMSAclinicalstudydemonstratedthattheLoopSystemcouldaccuratelymonitorrespirationrateaswellasthepredicate,withinthesensorrangeArterialhemoglobinoxygensaturation(SpO2)FundamentalScientificTechnologySpO2measuredbyanalyzingreflectanceofcertainLEDfrequenciesinaphotoplethysmogramdesign.
Thediodesaremountedinthedevicesuchthattheyareincontactwiththeskin.
SameTechnologicallyequivalentK181352:LoopSystemTraditionalPremarketNotificationPage5PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceSpO2Range70–100%70–100%IdenticalSpO2Accuracy2%ARMS,nomotion3%ARMS,nomotionBothcomplywithISO80601-2-61aswellaswithFDAGuidanceforPulseOximeters(2013)HeartRate(HR)FundamentalScientificTechnologyHRmeasuredbyanalyzingcyclicvariationsinreflectanceofcertainLEDfrequenciesinaphotoplethysmogramdesign.
Thediodesaremountedinthedevicesuchthattheyareincontactwiththeskin.
SameTechnologicallyequivalentHeartRateRange25–240beatsperminute(BPM)25–250beatsperminute(BPM)Clinicallyequivalent.
BothcomplywithISO80601-2-61HeartRateAccuracy3BPMARMS,nomotion3BPMARMS,nomotionClinicallyequivalent.
BothcomplywithISO80601-2-61StandardsComplianceIEC60601-1IEC60601-1-2IEC60601-1-11ISO80601-2-61ISO10993-1IEC60601-1IEC60601-1-2IEC60601-1-11IEC60601-1-6ISO80601-2-61ISO10993-1BothdevicesmeetlatestguidelinesforsafetyandefficacyDesignUserInterfaceFingerclampWristbandBothdevicesareworntoprovidedeviceaccesstoperipheralarteries.
Differenceinparticularuserinterfacedoesnotraisenewquestionsofsafetyorefficacy.
K181352:LoopSystemTraditionalPremarketNotificationPage6PredicateDeviceMasimoMightySatRxFingertipPulseOximeterK181956ApplicationDeviceSpryHealth,Inc.
LoopSystemImpactonSubstantialEquivalenceWearinglocationandfrequencyForintermittentwearing,aka"spotchecking"Continuouswearing,nearlycontinuousrecordingofalldata.
Differenceinwearinglocationonthebodyandfrequencydoesnotraisenewquestionsofsafetyorefficacy.
Bothlocationsallowformonitoringperipheralarteries.
DisplayOLEDcolordisplayofHRandSpO2whendevicewornLEDcolordisplayofHRandSpO2ondemandSimilarPowersource2AAbatteriesInternalrechargeablebatteriesBothbattery-poweredDataCommunicationWireless(Bluetoothpairing)withmobiledevicessuchassmartphonesWireless(cellularconnection)viachargingstationtoSpryServer.
Bothdevicesaredesignedtotransmittheirdatatoalternatedevicesorsites.
TypeofprotectionUnknownTypeBF–AppliedPartperIEC60601-1ApplicationdevicemeetslatestguidelinesforelectricalsafetyPatientcontractingmaterialsPlasticPlasticSimilarBiocompatibilityComplianttoISO10993-1ComplianttoISO10993-1IdenticalK181352:LoopSystemTraditionalPremarketNotificationPage7F.
SummaryofSupportingDataSpryHealthhasconductedextensivetestingtoensurethattheLoopSystemmetdesignspecifications,functionsasintended,andconformstointernationallyrecognizedstandardsandFDAGuidelines.
BenchTestingAlltestresultsdemonstratetheperformanceofSpryHealthLoopSystemmettherequirementsofitspre-definedacceptancecriteriaandintendeduse.
Theresultsofthebench(non-clinical)testingdemonstratethattheLoopSystemisassafeandeffectiveasthepredicatedevice.
BenchtestingdemonstratedtheaccuracyoftheheartratemonitoringwiththeLoopSystem.
ThistestingwasconductedinaccordancewithISO80601-2-61Medicalelectricalequipment-Part2-61:ParticularrequirementsforbasicsafetyandessentialperformanceofpulseoximeterequipmentandinaccordancewiththeFDAGuidelinesforPulseOximeters-PremarketNotificationSubmissions[510(k)s]:GuidanceforIndustryandFoodandDrugAdministrationStaff(2013).
TheLoopSystemwasfoundtobeincompliancewithbothdocuments.
Additionally,transittestingandone-yearshelflifetestingwasconducted.
ElectricalSafetyandElectromagneticCompatibilityTestingElectricalsafetytestingwasconductedinaccordancewith:IEC60601-1:2005+AM1:2012Medicalelectricalequipment-Part1:GeneralrequirementsforbasicsafetyandessentialperformanceIEC60601-1-2:2014:MedicalElectricalEquipment–Part1-2:GeneralRequirementsforSafety–Collateralstandard:ElectromagneticDisturbances–RequirementsandtestsIEC60601-1-11:2015Medicalelectricalequipment–Part1-11:Generalrequirementsforbasicsafetyandessentialperformance–CollateralStandard:RequirementsformedicalelectricalequipmentandmedicalelectricalsystemsusedinthehomehealthcareenvironmentIEC60601-1-6:2013Medicalelectricalequipment–Part1-6:Generalrequirementsforbasicsafetyandessentialperformance–Collateralstandard:UsabilityTheSpryHealthLoopSystempassedallelectricalsafetyandEMCtesting.
BiocompatibilityTestingBiocompatibilitytestingwasconductedinaccordancewithISO10993-1:2009/AC:2010,Biologicalevaluationofmedicaldevices–Part1:Evaluationandtestingwithinariskmanagementprocess,andFDA'sguidancedocuments,UseofInternationalStandardISO10993-1,"Biologicalevaluationofmedicaldevices-Part1:Evaluationandtestingwithinariskmanagementprocess"issuedJune16,2016.
ThistestingdemonstratesthatthematerialsintheLoopBandwillnotcauseanadversebiocompatibilityreactionwhenusedasintended.
K181352:LoopSystemTraditionalPremarketNotificationPage8ClinicalStudiesTwoclinicalstudieswereconductedtodemonstratetheperformanceoftheLoopSystem.
Oneclinicalstudyinvestigatedtheaccuracyofrespirationratemonitoringin12healthyadultsubjectswitharangeofskintypesandrespiratoryrates.
TheLoopSystemaccuracywasshowntobeequivalenttoagoldstandardmonitoringprocedure.
Anotherclinicalstudyinvestigatedtheaccuracyofthepulseoximetrymonitoringin12healthyadultsubjectswitharangeofskintypes.
TheLoopSystemaccuracywascomparedtothegoldstandard,arterialbloodgasanalysis.
ThistestingwasconductedinaccordancewithISO80601-2-61Medicalelectricalequipment-Part2-61:ParticularrequirementsforbasicsafetyandessentialperformanceofpulseoximeterequipmentandinaccordancewiththeFDAGuidelinesforPulseOximeters-PremarketNotificationSubmissions[510(k)s]:GuidanceforIndustryandFoodandDrugAdministrationStaff(2013).
TheLoopSystemwasfoundtobeincompliancewithbothdocuments.
G.
ConclusionSpryHealthconcludesthattheapplicationSpryHealthLoopSystemissubstantiallyequivalenttotheMasimoMightySatRxFingertipPulseOximeter(K181956).
ThedeviceshaveasimilarIndicationsforUse,features,technologyandaccuracyinmonitoringrespirationrate,heartrateandpulseoximetrysaturation.
- Servicesrewrite相关文档
- sectionrewrite
- strategiesrewrite
- discrepanciesrewrite
- dependentrewrite
- relationrewrite
- joinedrewrite
digital-vm$80/月,最高10GDigital-VM1Gbps带宽带宽
digital-vm在日本东京机房当前提供1Gbps带宽、2Gbps带宽、10Gbps带宽接入的独立服务器,每个月自带10T免费流量,一个独立IPv4。支持额外购买流量:20T-$30/月、50T-$150/月、100T-$270美元/月;也支持额外购买IPv4,/29-$5/月、/28-$13/月。独立从下单开始一般24小时内可以上架。官方网站:https://digital-vm.com/de...

香港CN2云服务器 1核 2G 35元/月 妮妮云
妮妮云的来历妮妮云是 789 陈总 张总 三方共同投资建立的网站 本着“良心 便宜 稳定”的初衷 为小白用户避免被坑妮妮云的市场定位妮妮云主要代理市场稳定速度的云服务器产品,避免新手购买云服务器的时候众多商家不知道如何选择,妮妮云就帮你选择好了产品,无需承担购买风险,不用担心出现被跑路 被诈骗的情况。妮妮云的售后保证妮妮云退款 通过于合作商的友好协商,云服务器提供2天内全额退款到网站余额,超过2天...
易探云2核2G5M仅330元/年起,国内挂机宝云服务器,独立ip
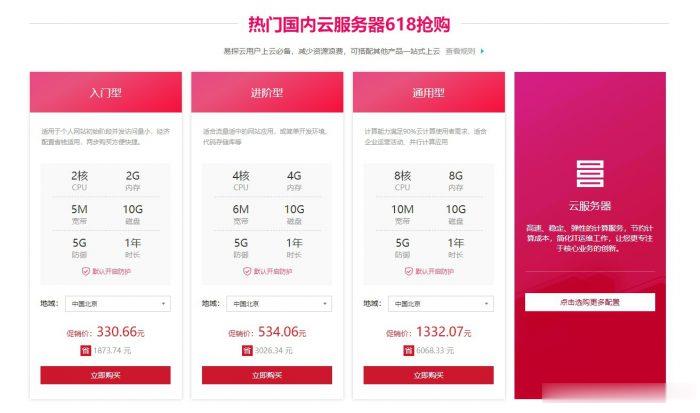
易探云怎么样?易探云是国内一家云计算服务商家,致力香港服务器、国内外服务器租用及托管等互联网业务,目前主要地区为运作香港BGP、香港CN2、广东、北京、深圳等地区。目前,易探云推出深圳或北京地区的适合挂机和建站的云服务器,国内挂机宝云服务器(可选深圳或北京地区),独立ip;2核2G5M挂机云服务器仅330元/年起!点击进入:易探云官方网站地址易探云国内挂机宝云服务器推荐:1、国内入门型挂机云服务器...
rewrite为你推荐
-
金士顿内存真假如何辨别KINGSTON 内存真假锦天城和君合哪个好和君咨询(王明夫为董事长)到底怎么样?有人说很好,空间大;也有人说像待遇差。音乐播放器哪个好目前音质最好的音乐播放器录音软件哪个好有什么录音软件好用??苹果手机助手哪个好苹果手机助手哪个好用些谁知道dnf魔枪士转职哪个好dnf魔枪士专职哪个行车记录仪哪个好行车记录仪什么牌子好如何增加百度收录如何快速提高百度收录量dns服务器故障DNS服务解析故障 怎么办360云盘下载360云盘和百度云哪个好用,请说出为什么。