characterized8090lu.com
8090lu.com 时间:2021-03-18 阅读:()
REVIEWThedynamicinterdependenceofamebiasis,innateimmunity,andundernutritionHansP.
Verkerke&WilliamA.
PetriJr.
&ChelseaS.
MarieReceived:17April2012/Accepted:21September2012/Publishedonline:1November2012#Springer-VerlagBerlinHeidelberg2012AbstractEntamoebahistolytica,theprotozoanparasitethatcausesamebicdysentery,greatlycontributestodiseaseburdeninthedevelopingworld.
Effortstoexhaustivelycharacterizethepathogenesisofamebiasishaveincreasedourunderstand-ingofthedynamichost–parasiteinteractionandtheprocessbywhichE.
histolyticatrophozoitestransitionfromgutcom-mensalstoinvadersoftheintestinalepithelium.
Mousemod-elsofdiseasecontinuetobeinstrumentalinthisarea.
Atthesametime,large-scalestudiesinhumanpopulationshaveidentifiedgeneticandenvironmentalfactorsthatinfluencesusceptibilitytoamebiasis.
Nutritionalstatushaslongbeenknowntogloballyinfluenceimmunefunction.
Soitisnotsurprisingthatundernutritionhasemergedasacriticalriskfactor.
AbetterunderstandingofhownutritionalstatusaffectsimmunitytoE.
histolyticawillhavedramaticimplicationsinthedevelopmentofnoveltreatments.
Futureworkshouldcontinuetocharacterizethefascinatinghost–parasitearmsracethatoccursateachstageofinfection.
KeywordsEntamoebahistolytica.
Amebiasis.
Innateimmunity.
UndernutritionIntroductionEntamoebahistolyticaisaprotozoanparasiteendemictomuchofthedevelopingworld.
TheWHOestimatesthat100millionyearlyE.
histolyticainfectionsresultinapproximately100,000deathsfromdysentery,colitis,andextraintestinaldiseasessuchasamebicliverabscess(ALA).
Amebiasisspreadsbyingestionoffoodandwatercontaminatedwithamebiccysts,whichareresistanttothecausticenvironmentofthestomachandpassunimpededintotheintestine.
Excystationandcolonizationoccurinthetermi-nalileum.
E.
histolyticatypicallyestablishesacommensalrelationshipwiththehostandcystsofnonpathogenictroph-ozoitesareexcreted,perpetuatingthelifecycleoftheparasite.
Itisestimatedthat90%ofcasesareasymptomaticwhile10%ofinfectionsprogresstosymptomaticdisease.
Asmallbutsignificantsubpopulationofsymptomaticindividualsdevel-opssevereextraintestinalamebiasis,includingamebicliverabscess[1,2].
IthaslongbeensuspectedbutisnowconfirmedthatundernutritionsignificantlyincreasessusceptibilitytoE.
histolyticainfection.
Undernutritioncontributesto2.
2mil-liondeathsand21%ofdisability-adjustedlifeyearsinchildrenunder5.
Estimatesarethatundernutritionanditsassociateddiseasescontributeto35%ofchilddeathsand11%oftheglobaldiseaseburden[3].
StudiesofamebiasisinhumanpopulationsandmousemodelshaveinformedourknowledgeofhostfactorsthatinfluencesusceptibilitytoE.
histolyticaandparasitefactorsthatmaydirectlyalterviru-lence.
AdaptiveimmunitydevelopsincontinuallyexposedhumanpopulationsandisbelievedtomainlyresultfromproductionofneutralizingantibodiestargetedtotheGal/GalNAcadherencelectin[4].
SecretoryIgAagainstthislectinisassociatedwithprotectioninhumans[5]andhasrecentlybeenshowntocorrelatewithimmunityinexperi-mentallyvaccinatedbaboons[6].
Studiestargetingvaccinedevelopmentinmicesuggestthatprotectiveimmunityismediatedbyaninterferongamma(IFN-γ)-dependentTcellresponseduringinfectionwithE.
histolyticatrophozoites[7,8].
Asvaccinedevelopmentisongoing,thisreviewfocusesontheinnateimmuneresponseduringamebiasis.
WeThisarticleisacontributiontothespecialissueonImmunoparasitology-GuestEditor:MiguelStadeckerH.
P.
Verkerke:W.
A.
PetriJr.
(*):C.
S.
MarieDivisionofInfectiousDiseasesandInternationalHealth,UniversityofVirginiaHealthSystem,MR6buildingRoom1107,29815thSTSW,Charlottesville,VA22903,USAe-mail:wap3g@virginia.
eduSeminImmunopathol(2012)34:771–785DOI10.
1007/s00281-012-0349-1emphasizetheimportanceofenvironmentalfactorsthatalterhostresistancetoamebicdisease,inparticulartheemergingroleofhostnutritionalstatus.
WealsoexplorerecentworkthathasshedlightonbothhostandparasitegeneticfactorsimportantinthepathogenesisofE.
histolyticainfection.
ParasiteKnownamebicvirulencefactorsincludetheGal/GalNAcadherencelectin,cysteineproteases(CPs),arginase,ameba-pores,alcoholdehydrogenase,peroxiredoxin,cyclooxyge-nase2,andlipopeptidophosphoglycan(LPPG)[9,10].
StudiesofE.
histolyticavirulencecontinuetouncovernewfactorsinvolvedinpathogenesisandinteractionwiththehostinnateimmunesystemandmorearelikelytoemergefromfurtheranalysisoftheparasitegenome[11].
Charac-terizedvirulencefactorsofE.
histolyticaareoftendown-regulatedinnonpathogenicisolates[12,13]orabsentinthenonpathogenicamebicspeciesEntamoebadisparandEnt-amoebamoshkovski[14–16]RecentworkinE.
histolyticagenomicssupportsarolefortheparasitegenotypeandgeneticrecombinationintheprogressionofamebiasis.
Alietal.
havefoundthattroph-ozoitesisolatedfromthegutandliverofpatientswithbothintestinalandextraintestinaldiseasearegeneticallydistinctfromparasitesinthestool[17].
Oneexplanationisthatgeneticallydistinctsubpopulationsofvirulenttrophozoitesexistinthegut.
Alternatively,progressionofamebicdiseasemaycorrespondtogeneticreorganizationeventssuchasrecombinationthatpromoteaninvasivephenotype.
Howev-er,thehostandparasiteeffectorsdrivingtheseeventsateachstageofinfectionremainpoorlydefined.
Developingmorepowerfultoolstoperformwithin-patientcomparisonsofamebicvirulenceatdifferentstagesofinfectionwillgreatlyincreaseourunderstandingofthisprocess.
ItalsoremainsunclearwhetherregionalgenomicdifferencesinE.
histolyticastrainsaffectpathogenicity.
Gilchristetal.
compellinglydemonstratedthatexpressionofvirulencegenesisregulatedatthemRNAlevelinthefirstgenome-wideanalysisoftheE.
histolyticatranscriptomein2006.
ThisstudydemonstratedthatinvasivetrophozoitesinmicesignificantlyalteredmRNAexpressionforgeneswithdiverserolesinmetabolismandvirulence[18].
Workonthecalcium-regulatedtranscriptionfactorURE3BPalsosup-portsacrucialrolefortranscriptionalregulationinamebicvirulence.
URE3BPwasoriginallyidentifiedasaregulatoroftheGal/GalNAcadherencelectinandferredoxin.
Laterworkidentifiedacalcium-dependentphospholipid-associatedpeptide,ehC2A,whichseques-tersURE3BPtotheplasmamembraneinhighcalcium[19].
AcomprehensivestudyoftheroleofURE3BPinamebicvirulencehasrevealedthattrophozoitesexpressingadominantpositiveformofURE3BParemorepathogenic,formingdramaticallylargerlesionsintheliversofchallengedgerbils[20].
ThepotentcytotoxiceffectthatE.
histolyticaexertsonhostcellsisevidencethattheparasitepossessesauniquearmamentariumofvirulencefactors.
However,muchworkremainstobedonetodefineparasitefactorsandhowtheyinteractwiththehostateachstageofdisease.
Furtherclarifyingthemechanismsbywhichtrophozoitestransitionfromintestinalcolonizerstoinvasivepathogenshaspoten-tialforfutureanti-parasiticdrugdevelopment.
EnvironmentDiarrhealdiseaseslikeamebiasisareendemictothede-velopingworldwhereanumberofenvironmentalfactorsincludinglimitedaccesstocleanfoodandwater,andexposuretootherenteropathogenscontributetoincreasedriskofinfection[1,3,16,21–23].
Wearenowinthe14thyearoftrackingthemedicalhistoryofacohortofchil-drenlivinginMirpur,anurbanslumofDhaka,Bangla-deshwhereE.
histolyticainfectionisoneofthemajorcausesofdiarrhealillness.
SomeofthemajorfindingsfromthispopulationaresummarizedinTable1.
Apicturehasbeguntoemergeinwhichinfantundernutritionandstuntingpredisposesyoungchildrentoamebiasis,whichinturnexacerbatespreexistingnutritionalimbalancebyalteringgutfunction.
Undernutritionincreasestheriskofdiarrheaanditsas-sociatedmortality[24].
UndernourishedchildrenhavehigherratesofinfectionbyprotozoanparasitesparticularlyE.
histolyticaandCryptosporidiumrelativetowell-nourishedcontrols[25].
ArecentstudyofinfantsfromMirpurinthefirstyearoflifebyMondaletal.
showedthatundernutritionandstuntingat12monthsofageissignifi-cantlyassociatedwithnutritionalstatusatbirth,durationandseverityofdiarrhealepisodes,anddiminishedintestinalbarrierfunction[26].
Whilethisassociationisquiteclear,thecomplexetiologyisnotyetfullyunderstood.
Proteinenergymalnutritionisthemostcommoncauseofhumansecondaryimmunodeficiency,whichisparticularlyproblematicfordiarrhealdiseasesthatfurtherdiminishnu-trientuptakefromthegastrointestinaltract[27–30].
Acutestarvationinitiatesenergy-savingmechanisms,shuntingpowerawayfromimmunefunction.
Chronicnutritionaldeprivationleadstosevereimmunedeficitsexemplifiedbythedramaticthymicatrophyobservedincadaversofunder-nourishedindividuals[31,32].
Complementdeficits,re-ducedIgAproductionandspecificity,alteredgutbarrierfunctionduetorestructuredmicrovilli,impairedphagocyticcapacity,anddiminishedoxidativebursthaveallbeende-scribedasconsequencesofenergydeficitintheimmune772SeminImmunopathol(2012)34:771–785Table1SummaryofstudiesfromMirpuramebiasisbirthcohortAuthorDateStudyMajorfindingsHaqueetal.
2001MeasuredstoolIgAanti-lectinandassociationwithincidenceofEntamoebahistolyticadiarrheaMucousalIgAantibodiesraisedagainsttheamebicGalNAc-lecticareprotectiveagainstE.
histolytica.
Corroboratedin2006Haqueetal.
2002Anti-lecticserumIgGIsolatedtrophozoitesweregeneticallydiverseAnti-lecticfecalIgALackofserumIgGagainsttheGalNAclecticlinkedtoresistancePCRanalysisbyisolatedtrophozoites86%reductioninnewinfectionof12monthsinchildrenwhodevelopedIgAanti-lectinHaqueetal.
2003DiagnostictoolstomeasurecausesofdiarrhealepisodesWell-nourishedchildrenhadfewerepisodesofdiarrhealoverallMostfrequentcausesofdiarrheaincludedenterotoxigenicEscherichiacoli,Shigella,Rotavirus,Giardialamblia,Cryptosporidiumparvum,andE.
histolyticaE.
histolyticacontributedtooverallmorbidityfromdiarrhealillnessDuggaletal.
2004GenotypeleukocyteantigenclassIIallelesChildrenwithDQB1*0601/DRB1*1501haplotypewere10.
1timesmoreresistanttoE.
histolyticaandlesslikelytobesero-positiveforanti-lectinIgGHLAclassII-restrictedimmuneresponsesprotectiveagainstE.
histolyticainfectionTarletonetal.
2006VerbalandnonverbaltestforcognitiveabilityCognitivescorenegativelyassociatedwithstuntinginschool-agechildrenE.
histolyticadiarrheawasnotindependentlyassociatedwithcognitivedeficitswhenthestudywascontrolwithsocialfactorsMondaletal.
2006WeightforageZ-scoreE.
histolytica-associateddiarrhealillnesswasnegativelyassociatedwithgrowthofpreschoolchildrenasmeasuredbyWHOWAZandHAZHeigthforageZ-scoreCryptosporidiumandGiardiadiarrhealepisodesnotassociatedwithgrowthStoolantigendiagnosticsforCryptosporidiumandE.
histolyticaMicroscopicdiagnosicsforGiardiaHaqueetal.
2007IFN-γproductionbyamebicantigenstimulatedPBMCsAbove-averagesecretionofIFN-γbyPBMCsassociatedwithlongersurvivalwithoutE.
histolyticadiarrhealepisodesAdjustedforstunting,theincreasedriskremainedmildlysignificantConcludedthatIFN-γproductionlinkedtonutritionalstatusisprotectiveMondaletal.
2009AnalyzedassociationbetweenE.
histolyticadiarrheaandundaernutritionEnterotoxigenicE.
coli,Cryptosporidium,andE.
histolyticainfectionmorecommoninmalnourishedchildrenMalnutritioncontributestosusceptibilityinasubsetofentericinfectionsPetersonetal.
2010TNF-αproductionbyamebicantigenstimulatedPBMCsHighlevelofTNF-αproductionassociatedwithincreaseriskofthefirstandrecurrentE.
histolyticadiarrhealepisodesMicroarrayanalysisofwholebloodandcolonbiopsysamplesfrompatientsinacuteandconvalescentstagesofamebiasisSymptomaticinfectionwasassociatedwithsignificantlyhigherproductionofTNF-αproteinandmildlyelevatedTNF-αmessagelevelsDuggaletal.
2011MeasuredgeneticvariantsinleptinandtheleptinreceptorandinvestigatedtheirassociationwithamebiasisChildernhomozygousforaSNPencodingasingleaminoacidsubstitutionintheleptinreceptor(Q223R)werefoundtobenearlyfourtimesmorelikelytohaveaninfectioncomparedwiththosehomozygousforthewild-typealleleAdultALApatientswerelesslikelytocarrytheprotectiveglutaminealleleMicecarryingtheprotectivealleleweresignificantlymoreresistanttoexperimentalamebiasisPetersonetal.
2011MicroarrayanalysisofintestinalbiopsiesinacuteandconvalescentamebiasisREG1AandREG1BwerehighlyupregulatedinhumanbiopsiesfrompatientswithacuteversusconvalescentamebiasisRT-qPCRforREG1A/1BResultconfirmedbyRT-qPCRChallengedREG1knockoutmicewithE.
histolyticatrophozoitesREG1knockoutmiceweresignificantlymoresusceptibletoexperimentalamebiasisSeminImmunopathol(2012)34:771–785773system(reviewedin[33]).
Globalsuppressionanddysregu-lationofimmunefunctionassociatedwithacuteandchronicundernutritionmaybethemostimportantindependentpre-dictorofE.
histolyticadiarrheainthefirstyearoflife.
Reciprocally,E.
histolyticadiarrhealepisodesincreasetheriskofbecomingundernourished[23,26,34,35].
Chil-drenwithE.
histolytica-associateddiarrheaarenearlythreetimesmorelikelytobeclinicallyundernourishedandareatdramaticallyincreasedriskofstunting[34].
Childhoodundernutritionanddiarrheaareinextricablylinkedwithprofoundconsequencesfortreatmentout-comesinthedevelopingworld.
Intestinalinflammationresultingfromrepeatedexposuretoentericpathogensleadstomalabsorptionofcriticalnutrients,whilesecond-aryimmunodeficiencyrelatedtocompromisednutritionalstatusmakeschildrenunder5particularlyvulnerabletothedevastatingcycleofundernutrition,disease,andpov-erty.
Tobreakthiscycle,aholisticapproachisneeded.
TreatmentstrategiestotargetE.
histolyticadiarrheainthecontextofanundernourishedpatientmustbedesigned.
Morebroadly,nutritionalsupplementationshouldbeenrichedtoprovideessentialnutrientsaffectedbyrepeat-edexposuretoenteropathogensandsuchsupplementscouldbecomearoutinepartoftreatment.
Finally,suc-cessfulvaccinesmayutilizeadjuvantsthatstimulatein-natefactorsdeficientinundernourishedindividuals.
Whiletherapeuticandnutritionalinterventionsarecritical,humangeneticfactorsthataffectinnateresistancetoE.
histolyticainfectionfurthercomplicatetheinteractionbe-tweennutritionalstatusandparasiticdisease.
HostNaturalimmunityinhumansMountingevidencesuggeststhathostgeneticfactorsinflu-encefrequencyandseverityofE.
histolyticadiarrhealepisodes.
TheHLAclassIIalleleDQB1*0601andthehaplotypeDQB1*0601/DRB1*1501areassociatedwithdrasticallydecreasedratesofE.
histolyticainfection[36].
Morerecently,a12-yearstudyoftheMirpurcohorthasshownthatasingleaminoacidreplacement(Q223R)intheextracellulardomainoftheleptinreceptorencodedbyaSNPisassociatedwithanearlyfourfoldincreaseinsuscep-tibilitytoinfectionbyE.
histolyticaaswellasanoveralldecreaseintimetoinfection.
TheeffectsofthemutationwereconfirmedinapopulationofadultmaleALApatients,wherehomozygosityforthealleleencodingarginineatposition223wasassociatedwithtwofoldincreasedsuscep-tibilitytoextraintestinaldisease.
Theseobservationshigh-lighttheimportanceofleptinsignalingandpointtoaspecificmutationthatincreasessusceptibilitytoE.
histoly-tica,decreasestimetoinfection,andincreasesthelikelihoodofdevelopingextraintestinalamebiasis[37].
Itisclearthatthehostgeneticbackgroundinfluencessusceptibilitytoamebiasis.
However,environmentalfactorscomplicatethepicturebysimultaneouslyaffectingthephe-notype.
Itisimportanttodelineateprimaryresistancedeter-minedbygeneticsandsecondaryresistancedeterminedbynutritionalstatusandotherenvironmentalfactors.
Itiscrit-icaltoidentifymorehostgeneticfactorsthataffectE.
histolyticainfection.
Thesestudieswillnotonlyincreaseourunderstandingofthepathogenesisofamebiasisbutalsoaidintargetingtherapiestoat-riskpopulations.
CytokinesTNF-αTNF-αisacytokinewithdiverserolesinimmunity.
Dependingonthefactorsrecruitedtoligand-boundTNFR1/2,TNF-αcanstimulategrowthanddifferentiationthroughMAPK,survivalandinflammationthroughNFκB,orcelldeaththroughcaspaseactivation[38].
TheamebiclectininducesTNF-αproductionbymacrophages[39]andTable1(continued)AuthorDateStudyMajorfindingsMondaletal.
2012Analysisofassociationbetweenmalnutrition,socioeconomicstate,immunefactorandamebiasisinthefirstyearoflifeChildrenmalnourishedatbirthweresignificantlymoresusceptibletoE.
histolytica,Cryptosporidium,andETECinfectionsMalnutritionin12monthsispredictedbyprolongeddiarrhealepisodes,intestinalbarrierdysfunction,maternaleducation,andfamilyincomeConcludedthatmalnutritionatbirthpredisposeschildrentoamebiasiswhichaltersintestinalbarrierfunctionandinfluencesnutritionalstatusat12monthsHere,wedescribemajorfindingsfromthelong-termprospectivestudyofapopulationlivinginMirpur,anurbanslumofDhaka,Bangladesh.
Childrenwereobservedeveryotherday.
DiarrhealstoolsweretestedforE.
histolyticausingantigendetectionandPCR.
Bloodwasdrawnevery4monthsfordetectionofserumantibodiesandcytokineresponse,andmonthlystoolsampleswereobtainedtomeasuresecretoryimmunoglobulinAresponse.
Anthropometricswereassessedevery4months774SeminImmunopathol(2012)34:771–785TNF-αactsasapotentchemoattractantforE.
histolytica[40];however,theroleofTNF-αinamebiasisisnotentirelyclear.
Invitro,TNF-αenhanceskillingofE.
histolyticatroph-ozoitesbyneutrophilsandmacrophages[41,42].
Invivo,TNF-αseemstoexacerbatedisease.
BlockingTNF-αpro-ductioninthehumanxenographSCIDmousemodelofamebiasisreducesinflammationandintestinaldamage[43].
Thisfindingwasrecentlyextendedtohumanpopula-tionswhereTNF-αproductionbyamebicantigenstimulat-edPBMCscorrelatedwithincreasedsusceptibilitytoE.
histolyticadiarrhea[44].
IthasbeensuggestedthatIFN-γandprostaglandinE2modulateTNF-αproductionduringinfection[45].
Thus,acomplexarrayoffactorslikelydeter-mineswhetherTNF-αhasaprotectiveordestructiveroleduringamebicinfection.
IFN-γAnalysisofcytokineprofilesintheMirpurstudypopulationhasalsoshownthataboveaverageproductionofIFN-γbutnotIL-5byamebicantigenstimulatedPBMCswasassociatedwithfavorableoutcomesanddecreasedincidenceofE.
histo-lyticainfection.
Whencontrolledfornutritionalstatus,theobservationremainedonlymildlysignificantsuggestingthatIFN-γproductionwasdampenedinmalnourishedsubjects[46].
TheimportanceofIFN-γhasbeenrecapitulatedinmurinevaccinationstudies,whichhavedemonstratedthatIFN-γisasignificantcorrelateofprotectionforE.
histolytica[7,8].
IL-10IL-10-deficientmicearemoresusceptibletoamebicinfec-tion[47],andundernutritionmodifiesIL-10production[48]perhapscontributingtoincreasedsusceptibilityofunder-nourishedindividualstoE.
histolyticadiarrhea.
Overall,IL-10islikelytoplayaroleinconnectingnutritionalstatustoamebiasis.
LeptinTheadipocytokineleptinlinksnutritiontoimmunity.
Im-munefunctionsareimpairedandleptinlevelslowinunder-nourishedindividuals[49].
However,theroleofleptininstarvation-inducedimmunedysregulationhasnotbeenfullyelucidated.
Datafromhumanstudiesintotheeffectsofleptindeficiencyonimmunityinmalnourishedchildrenarecontradictory[50,51].
Nonetheless,miceandhumanswithimpairedleptinsignalingexhibitcompromisedim-munefunctionandaremoresusceptibletomanyinfections,includingamebiasis[52,53]Leptinsignalsthroughthelongformofitsreceptorviathereceptor-associatedJanuskinase2(JAK2).
JAK2phosphorylatesintracellulartyrosineresiduesY985,Y1077,andY1138,whichrecruitSHP2,STAT5,andSTAT3,respec-tively.
SHP2positivelyregulatestheERK/c-fospathway[54].
ActivatedSTAT3upregulatesSOCS3,whichservesasbothafeedbackinhibitorofleptinsignalingandarepressorofapo-ptoticpathways.
Leptinreceptor-mediatedSOCS3signalingalsoregulatesanumberofproinflammatorypathways[55]Leptinsignalingplaysacrucialandwell-characterizedroleinanumberofimmunepathwaysthatareimportantforpro-tectionagainstamebiasis.
LeptinenhancesCCLchemokinesecretioninmurinemacrophages[56],whichpromotesche-motaxisofmonocytes.
Leptinalsoactsdirectlytomobilizemonocytepopulationsvialeptin-dependentPI3K/Aktactiva-tion[57].
Inleptinandleptinreceptor-deficientmice,macro-phagesexhibitphenotypicvariationslikediminishedphagocyticcapacityandloweredcytokineproduction[58,59].
LeptinsignalssecretionofproinflammatorycytokinesTNF-αandIL-6[60]andmayregulatethemonocyticoxidativeburst[61].
Stimulationwithleptinupregulatesproductionofinterleukin1receptorantagonist[62]andIFN-γinducibleprotein[63].
Overall,leptincontributestoaTh1proinflammatoryimmuneresponse,whichiscrucialforclearanceoftrophozoitesduringamebiasis.
Leptinre-ceptornullmice(db/db)exhibitaskewedTh2cytokineprofileanddiminishedTcellproliferation[64],whichcor-relatewithincreasedsusceptibilitytoE.
histolytica.
ProinflammatorychemokinesandcytokinesIL-6,IL1-β,andCXCL1areupregulatedinresponsetoleptin[65],andleptinconcentrationsspikeduringgutinflammation[66].
Theprimaryactionofleptininintestinalepithelialcells(IECs)appearstobemediatedthroughSTAT3,atranscrip-tionfactorknowntoregulateepithelialhomeostasisduringinfectionandinflammation[67,68].
PathogenicstrainsofbacteriainmiceactivateSTAT3incolonicepithelialcells,initiatingaprotectiveTh17mucosalimmuneresponse,characterizedbysecretionofIL-17.
TH-17responsestim-ulatesproductionofIL-6andIL-8,potentmediatorsofinflammationandneutrophilchemotaxis,respectively[69].
Leptinstimulatesanti-apoptoticandproliferativepath-waysthatpromotebarrierfunctionandwoundhealinginepitheliallayers[66,69–73].
Leptinsignalingisanti-apoptoticinavarietyofcelltypes[74].
Dendriticcellsfromdb/dbmicearemorepronetoapoptosispotentiallyasaresultofdecreasedPI3K/Akt,STAT3,andIκB-αsignaling[75,76].
Increasedcaspase-3expressionisobservedincolonicepithelialcellsofdb/dbmice[69].
Thepleiotropicroleofleptininimmunefunctionandenergybalancesupportsthehypothesisthatleptiniscriticaltoanappropriateimmuneresponseinentericdiseaseslikeamebiasisandmayexplainmechanisticallythestrongasso-ciationbetweenundernutritionandamebicdisease.
RecentstudiesinhumansandmicehaverevealedtheimportanceofleptinsignalinginprotectionagainstE.
histolytica.
SeminImmunopathol(2012)34:771–785775Guoetal.
haveshownthatob/obanddb/dbmicearemoresusceptibletoamebicinfectionthanarewild-typecontrols.
Moreover,leptinsignalingintheintestinalepithe-liumalonewassufficienttoconferwild-typeresistance.
Thesiteofleptin-mediatedresistancetoE.
histolyticawasde-terminedusingtissuespecificdeletionsoftheleptinreceptorinE.
histolyticaresistantC57B2/6mice.
Deletionoftheleptinreceptorintheintestinalepitheliumwassufficienttorendermicesusceptibletoamebicinfection.
ExpressionofaleptinreceptordeficientinSTAT5signalingalsorescuedthesusceptiblephenotype.
However,complementationwithreceptorsdeficientinSHP-2orSTAT3didnotrestoreresis-tance,implicatingSTAT3andSHP2butnotSTAT5inleptin-mediatedresistancetoamebiasis[52].
Tofurtherunderstandleptinsignalinginhostdefense,wedevelopedanassaytomeasureamebiccytotoxicityinsinglecells.
Physiologicalconcentrationsofleptinwereprotectiveagainstamebiccytotoxicityincellsthatendog-enouslyorexogenouslyexpressedleptinreceptor.
InHEK293Tcellsexogenouslyexpressingleptinreceptorsig-nalingmutants,onlycellsdeficientintheleptin-dependentSTAT3signalingpathwaylostleptin-mediatedresistancetoamebicchallengewhileSHP2andSTAT5mutantsremainedresistant.
TheQ223Rmutationintheleptinreceptoralsoincreasedamebiccytotoxicityinvitro,andcellsexpressingtheQ223formoftheleptinreceptordisplayedsignificantlyhigherlevelsofactivatedSTAT3inresponsetoleptin[77].
Incombination,theseresultsimplythatleptin-dependentSTAT3activationmediatesdirectcellularresistancetoame-biccytotoxicitywhileleptin-activatedSHP-2signalingmaybeimportantincoordinatingtheglobalimmuneresponsetoamebiasis.
Itisimportanttoemphasizethatthismechanismwaselucidatedinmurineandinvitromodelsofamebicdiseaseandhasyettobeextensivelyvalidatedinhumans.
Innatecell-mediatedimmunitytoE.
histolyticaIntestinalimmunityTheintestinalepitheliumisessentialforprotectionagainstamebiasis.
Approximately90%ofE.
histolyticainfectionsarecommensal,suggestingthatthegutishighlyeffectiveatlimitingprogressiontosymptomaticdisease[78].
Thepro-tectiveroleoftheepitheliuminamebiasisismediatedbythemucousallayer,whichpreventsamebafromadheringtoIECs.
IECsandtightgapjunctionsprovideasecondphys-icalbarriertoinvasion.
Asthesebarriersbegintofail,epithelialcellsrecruitandactivateothereffectorsofinnateimmunitythroughchemokineandcytokinerelease.
Resi-dentmicrobesmayalsoaffectE.
histolyticavirulenceandintestinalinflammation.
AsimplifiedmodelofintestinalinvasionbyE.
histolyticatrophozoitesisdepictedinFig.
1.
MucousallayerMucusoffersthefirstlayerofprotectionagainstinvadingtrophozoitesintheintestine.
Gobletcellssecretemucins,whichsolubilizetoformagelatinousbarrierbetweenthelumenandepitheliumformingabarrieragainstentericpathogens.
StudiesbyChadeeetal.
haveshownthatsecret-edcolonicmucinsarecriticalforgastricmucosalrepair[79]andresistancetobacterialcolitis[80].
TheprocessbywhichinvasiveE.
histolyticatrophozoitespenetratetheprotectivemucousbarriertocontactandkillsub-mucousalepithelialcellsiswellunderstood.
TheGal/GalNacadherencelectinofE.
histolyticaadherestoglycosylatedcomponentsofthemucousalmatrix[81].
Next,parasiticcysteineproteasescleavemucinsatsitesoflowglycosylation.
Degradingmucinsandothercomponentsofthemucouslayerallowsamebatobindsub-mucousalcells,inducecelldeath,andpenetratetheepithelium[82].
RecentworkinMUC2knockoutmicehashighlightedthespecificimportanceofmucinsasprotectivemoleculesagainstE.
histolytica.
Bergstrometal.
demonstratedthatMUC2deficientmicechallengedwithE.
histolyticadis-playedanacuteproinflammatoryresponse,characterizedbyincreasedgrosspathology,luminalTNF-αandIFN-γ,andalteredexpressionofgapjunctionproteins[80].
ResultsfromChadeeetal.
recentlyrecapitulatedthisfindingincolonicloopsfromMUC2-deficientmiceandhavegoneontoshowthattrophozoitesalsorequireMUC2toinvadetheepithelium.
E.
histolyticaappearstoinducehigherMUC2expressionandtrophozoitesbecometrappedinthemucouscoatingofthecolonicepitheliumpresumablybe-foreinvading.
E.
histolyticacysteineprotease5(CP5)directlycleavesMUC2.
Expressionofthisproteaselikelyallowstheparasitetoovercometheprotectivemucousbarrier[83].
WhenCP5issilenced,trophozoitesfailtopenetratethecoloniclaminapropriaanddonotinduceproinflammatorycytokinesecre-tioninexvivomodelsofamebiccolonicinvasion[84].
TheseresultssuggestthatdisassociationofE.
histolyticafromthecolonicepitheliumbymucous,preventsadamag-inginflammatoryresponse.
MicrobiomeThemicrobesthatcolonizetheshallowsectionofthemu-cosallayeroftheintestinalepitheliummayimprovebarrierfunctionbyinhibitingadherenceofpathogenicspeciesandcompetingfornutrients.
Entamoebaarenaturallycolonizedbybacteria,whichaffectparasitevirulence[85].
Theaxeni-zationofEntamoebaaltersthesurfaceantigensofthepar-asite[86].
Astrophozoitesfeedprimarilyonresidentgutflora,itisplausiblethatdifferencesinhostmicrobialecol-ogycontributetotheinitiationofavirulentphenotype.
776SeminImmunopathol(2012)34:771–785Already,reductioninlactobacillusspecieshasbeenob-servedinALApatients[87].
Numerousstudieshaveshownthatmicrobialcomposi-tionislinkedtonutritionalstatusanditscompositioncanalternutrientabsorptionandbioavailability[88–91].
Nutri-entdeprivationaltershostsusceptibilitybutmayalsocon-tributetovirulenceoftheparasite.
Severalmicro-andmacronutrientshavebeenshowntoinfluenceE.
histolyticavirulenceincludingcalciumviaregulationofURE3BP[19],glucose[92],cholesterol[93],andiron[94].
Theemergingfieldofmicrobialmetagenomicsislikelytocontinuetorevealanimportantroleforthegutmicro-biomeinthepathogenesisofE.
histolyticaviamodulationoftheimmuneresponseandvirulenceoftheparasiteitself.
AntimicrobialcompoundsSecretedimmunoglobulins,defensins,andcathalecidinsre-inforcemucousalbarrierfunction,activelypreventingpath-ogeniccolonizationbyameba.
Cathelicidins,antimicrobialpeptidesresidentinthegutmucosa,areimportantmediatorsofmammalianinnateimmunityandwhileE.
histolyticatrophozoitesactivatecathelicidinproductioninbothhumanandmouseintestinalepitheliallayers,theyareresistanttotheircytolyticeffect.
AmebiccysteineproteasescleavethecathelicidinsLL-37andCRAMPbutthefragmentsretainbactericidalactivity[95].
Fourregeneratinggene(REG)C-typelectinshavebeencharacterizedinhumans.
Overall,REGsfunctiontoinducecellularproliferationandinhibitapoptosis.
REGIαseemstohaveaphysiologicalroleinthegastricmucosaandREGsarefoundthroughoutthegastrointestinalsystem.
REGIα,REGIβ,andREGIIImRNAsareoverexpressedinthecolonininflammatoryboweldisease,Crohn'sdisease,andulcerativecolitis[96].
Microarrayanalysisofintestinalbi-opsiesfrompatientsinearlyandrecoverystagesofamebi-asishasshownthatREGIαandREGIβarehighlyupregulatedduringacuteinfectionrelativetoconvalescenceandimmunohistochemicalstudiesconfirmthisresult[97].
Thus,REGslikelyhaveafunctionalroleinregenerationoftheintestinalepitheliumafteramebicdisease.
TherecentlydiscoveredantimicrobialfunctionofREGIII[98]suggeststhatREGproteinsmayhavedualfunctionsinintestinalbarrierreinforcementandamebickilling[99].
IntestinalepithelialcellsIECsrecognizemultipleamebicantigensanddirecttheimmuneresponseintheacutephaseofamebiasis.
Itisgenerallythoughtthattheepitheliumdownregulatespatternrecognitionreceptors(PRR)topreventchronicinflamma-tioninresponsetoresidentmicrobialantigens[9].
Interest-ingly,stimulationofIEClayerswiththeadherencelectinofE.
histolyticaincreasesexpressionofTLR2,whichrecog-nizesamebicLPPGandactivatesNFκB-regulatedsecretionofproinflammatorycytokinesfromhumanmonocytes,mac-rophages,anddendriticcells[100].
Invitro,whenstimulat-edwithamebicantigens,IECssecreteTNF-α,IL-6,GROa,andGM-CSF[101].
E.
histolyticaDNAhasbeenshowntoactivateTLR9[100]whichrecognizesunmethylatedCpGdinucleotides[101].
Additionally,CP5bindstoα(V)β(3)integrinonCaco-2coloniccellsviatheRGBmotifandstimulatesanNFκB-mediatedproinflammatoryresponsesviaPI3K/Akt[102,103].
IntheSCIDmouse–humanxeno-graftmodelofALA,amebicchallengeincreasedIL-8andIL-1βsecretionpresumablypromotingacuteneutrophilicinfiltration[104].
IECdeathitselfmayrecruitandstimulateimmunecells.
Ithaslongbeenthoughtthatcontact-Fig.
1Intestinalimmunity.
E.
histolyticatrophozoitesencounteralayeredhostdefenseastheyinvadetheintestine.
Intestinalepithelialcells(IECs)directaninflammatoryresponsetoinvasiveamebabyrecruitingneutrophils,macrophages,andotherimmunecells.
Trophozoitesthatsurvivemayprogresstothecirculatorysystemwheretheyencounterhumoralantibodiesandmustevadecomplement-mediatedlysisSeminImmunopathol(2012)34:771–785777dependentkillingbytrophozoiteswascanonicallyapoptotic.
Amebiccytotoxicityinducesmembraneblebbing,TUNELpositivity,surfaceexposureofphosphatidylserine,andcas-paseactivation.
Furthermore,caspaseinhibitionandoverex-pressionofanti-apoptoticeffectorslikeBcl-2areprotectiveinmousemodelsofdisease[105].
Pyropototicandnecroticdeathareincreasinglyrecognizedasimportantforamebiccytotoxicityashostcellsexperiencearapidlossofmembraneintegritynotconsistentwithcanonicalapoptosis.
Theselatterdeathpathwaysmaycontributemorepotentlytoinflammationintheintestinethroughreleaseofhostcellularcomponents.
AmebicinvasionthroughtheIEClayerandintothelaminapropriamayoccurpassivelythroughsmallgapsresultingfrominflammation[101].
Activepenetrationislikelymediatedbyamebicproteasesthatcleavegapjunc-tions,facilitatingtransepithelialmovement[14].
Interesting-ly,E.
histolyticahasadaptedtodampentheNFκB-mediatedinflammatoryresponseofIECsviainductionofheatshockprotein27(hsp27),whichsuppressesNFκBtranscriptionalregulation[106].
TheseobservationssuggestthattheintestinalepitheliumiscrucialbarriertoinfectionbyE.
histolytica.
PathogenicamebamustbreachthemucousalmatrixtoaccessIECs,whosePRRsrecognizeandrespondtoamebicinvasion.
Attheonsetofacuteamebiccolitis,ulcersformandinflam-matorysignalsfromIECsrecruiteffectorsofinnateimmu-nitytothesiteofdangerwhileinitiatinganadaptiveresponse.
Thedegreeofimmuneactivationintheintestinalepitheliumlikelydeterminestheprogressionandseverityofamebiasisasmuchoftheresultanttissuedamageismediat-edbythehostinflammatoryresponse.
Post-epithelialimmunityNeutrophilsTheprimaryroleofneutrophilsininnateim-munityistodestroyandengulfpathogensinaffectedtis-sues.
NeutrophilextracellularNADPHoxidasegeneratesreactiveoxygenspeciesthataretoxictoinvadingpathogens.
Neutrophilsalsoexpresssurfacereceptorsforopsoninsthatfacilitatephagocytosisofpathogensmarkedbyotherbranchesoftheimmunesystemfordestruction[115].
Neu-trophilsalsoexudeextracellulartraps(NETs)composedofchromatinandserineproteasesthatensnareandneutralizepathogens[116].
AroleforNETsinthepathogenesisofamebiasishasyettobecharacterized.
Trophozoitesthatbreachtheepithelialbarrierelicitarapidinfiltrationofneutrophilstothesiteofinfectioninearlystagesofintestinaldisease[107,108].
IECssecreteIL-8,apotentneutrophilchemoattractant,whenexposedtoE.
histolytica[109].
ActivationandtranslocationmayalsooccurinresponsetoanaphylotoxinsC5aandC3aofthecomplementsystemthoughamebicproteasescleaveandinactivatebotheffectors[110].
Itisgenerallyacceptedthatneutrophilsareresponsibleformuchofthetissuedamageassociatedwithamebiccolitis.
Whileneutrophilsdopossessamebacidalactivity,whichlike-lyaidsinpathogenclearanceforasymptomaticinfections,virulenttrophozoiteshaveadaptedeffectivemechanismsofresistanceandcounterattack.
NeutrophilsincultureamplifythecytopathogenicityofE.
histoltyicatrophozoitesonepithe-lialcells[111]likelyduetoneutrophildegranulationoftoxiccellularcomponents[112].
Leptindeficiencyandgeneticdifferencesinleptinsig-nalingpathwaysmayalsoaffectneutrophilresponsetoamebicinvasion.
Neutrophilsexpressashortformoftheleptinreceptorthatissufficientforleptinsignaling,whichinhibitsapoptoticpathwaysandactsasachemoattractant[54,113],andmaymodulateoxidativecapacityinneutro-philsduringinfection[114].
Thereissomeevidencefrommouseandinvitromodelsofamebiasisthatneutrophilsplayaprotectiveroleearlyininvasion[105,117,118].
NeutrophildepletionintheSCIDmousemodelexacerbatesintestinaldiseaseandliverab-scessformation[105,117,118].
However,theGR-1anti-bodiesusedinthesestudiesmayalsodepletemonocytesandeosinophils.
Inearlystagesofhepaticamebiasis,neutrophilsarethemajoreffectorsoftheacuteinflammatoryresponse,[119]butcanbebothprotective[120]anddestructive[111].
NeutrophilsprimedwithIFN-γ,TNF-α,orLPSdoexhibitamebicidalactivity[112].
However,E.
histolyticahasproventobefarmoreeffectiveatkillingneutrophils,withmorevirulentstrainskillingataratioof1trophozoiteto3,000neutrophils[112].
E.
histolyticatrophozoitesinduceneutrophilapoptosisdirectlybyengagingwithintegrinsandbyactivatingNADPHoxidase[121,122].
Invasiveamebicstrainsareresistanttotherespiratoryburstasanamebicperoxidoredoxinprotectstroph-ozoitesfromreactiveoxygenspecies[121,122].
Neutrophilcorpsesarequicklyphagocytosedbytrophozoites[112].
Figure2summarizessomeofwhatisknownabouttheroleofneutrophilsinhostdefenseagainstE.
histolytica.
MacrophagesMacrophagesplayimportantrolesinthepathogenesisofbothintestinalandhepaticamebiasis.
Macro-phagesareactivatedbyE.
histolyticatrophozoitesandareamebicidalwhenstimulatedwithCSF,IFN-γ,andTNF-α[112].
TheamebicsurfacecomponentLPPGactivatesaclas-sicalinflammatorymacrophageresponseuponTLR2/6andTLR4binding[123].
E.
histolyticaDNAalsopromotesmacrophageactivationbyTLR9binding[104].
Furthermore,macrophagesupregulateTLR2inresponsetotheamebicgalactose-specificlectin[104].
Macrophageamebicidalactiv-ityismediatedbynitricoxidesynthase(NOS)[124].
NOinhibitsimportantamebicvirulencefactorsincludingthecys-teineproteasesandalcoholdehydrogenase2[125].
Invasiveamebaareabletoregulatemacrophageresponsesinanumberofways.
Analogoustotheirinteractionwithneutrophils,trophozoitesinhibittherespiratoryburstof778SeminImmunopathol(2012)34:771–785macrophages.
Withmacrophages,thisfunctiondependsonanamebicarginasethatcompetitivelyconvertsL-arginine,asub-strateofmacrophageNOS,toL-ornithine[126].
Amebaalsomodulatemacrophagecytokinesecretion[127,128].
Produc-tionofTNF-αisblockedbyamebicdegradationofc-fosandTNF-αtranscripts[129].
E.
histolyticaproducesacyclooxy-genase2,theprostaglandinproductofwhichhashost-immunomodulatoryfunction[130,131].
Prostaglandintrig-gersacAMPspikeinmacrophages.
ThissignalactivatesPKAandIL-8secretion[131].
PKAinhibitsexpressionofTh1cytokinesandPKC-mediatedNOsynthesis[130].
Finally,themonocytelocomotioninhibitoryfactor(MLIF)ofE.
his-tolyticahasbeenimplicatedinalteringmacrophagefunction.
MLIFinhibitsNOandproinflammatorycytokineproductionwhilestimulatingthesecretionofIL-10,animportantanti-inflammatoryeffector[132,133].
Macrophagesrepresentasecondarylineofdefenseafterthemassiveinfiltrationofneutrophilsduringacutehepaticamebiasis.
NOSisprotectiveanditsliverspecificdeletionincreasesseverityofALAininfectedmice[124].
IFN-γisalsoimportantformacrophage-mediatedprotectionagainsttrophozoites.
BlockingIFN-γreceptorsincreasessusceptibil-itytoALAinmice[134].
Classicallyactivatedmacrophagesareeffectiveatclearingtrophozoitesandevenalternativeactivationcanpromotewoundrepairandrecruitmentofadap-tiveimmunecells[135].
E.
histolyticasuppressesbothacti-vatedclassesofmacrophages,underminingcell-mediatedimmunityandleadingtoestablishmentofchronicALA[127].
Figure3summarizessomeofwhatisknownabouttheroleofmacrophagesinhostdefenseagainstE.
histolytica.
NaturalkillerandnaturalkillerTcellsNaturalkillercellsdonotrequireprimingtobefunctionallycytotoxicagainstpathogens.
Theircanonicallymyopic"killer"functionhasbeenchallengedinrecentyearsasmorestudieselucidatediverserolesforNKcellsinimmuneregulation[136].
ActivatedNKcellssecreteIFN-γandTNF-α,importantfactorsinhost-immunitytoamebiasis.
NKcellsinfiltratetheliverduringtheacutephaseofexperimentalALAinmousemodels[137].
VirulenttrophozoitesstimulateNKcellcyto-toxicitymoreeffectivelythandonon-pathogenicstrains[138].
E.
histolyticamayregulateNKcellsthroughCP-mediatedcleavageofC5a,ananaphylotoxinknowntoacti-vateNKcellsduringsepsis[139].
NKTcellsexpressmarkersthatdelineateNKcellsbutco-expressαβTCR.
TheydifferfrommostT-cellpopulationsinthattheyrecognizeantigenspresentedbyCD1dinsteadofMHCassociatedantigens.
InvariantNKT(iNKT)cellsareasubsetofNKTcellsthatexpressinvariantα-TCRandproducelargeamountsofpro-andanti-inflammatorycyto-kinesuponactivation.
iNKTcellsareactivatedtoproduceIFN-γbutnotIL-4inaTLR/CD1d-dependentmanner[140].
StudiesinmousemodelsofALAsuggestthatclear-anceoftrophozoitesduringearlystagesofhepaticcoloni-zationisdependentonNKTcellactivity.
MicelackingNKTcellsarehighlysusceptibletoALA[141].
ClearancewasassociatedwithelevatedIFN-γproduction.
Likeneutrophils,NKTcellslikelyhaveacontext-dependenteffectondiseaseprogression.
TheirresponsetoamebicantigensmaycontributetopolarizingtheimmuneresponsetowardtheTh1phenotypethoughttobeprotectiveinamebiasis.
IntermsofALA,aftertheacutephaseofhepaticinfiltration,particularlyresistanttrophozoitessur-viveandexpand.
Atthisstage,iNKTcellsmayincreasetissuedamagebypropagatingdeleteriousinflammatorysig-nalsandstimulatingneutrophilchemotaxis.
Fig.
2NeutrophilsrecruitedtothesiteofE.
histolyticainfectiontargetpathogensbysecretingNADPHoxidase,anenzymethatcatalyzestheproductionofreactiveoxygenspecies.
Interleukins,interferons,andtumornecrosisfactoralphaprimeandrecruitmoreimmunecells.
Theparasiterespondswithperoxiredoxinandcysteineproteases,whichcleaveandneutralizehostfactorstomakewayforlectin-mediatedadherenceandeffectorsofamebiccytotoxicitySeminImmunopathol(2012)34:771–785779ComplementThepathogenesisofextraintestinalamebiasisdependsonevasionofhumoralinnateimmunitybyE.
histolyticaandbothalternativeandclassicalbranchesofthecomplementsystemareinvolved.
Allcomplementpath-waysconvergetoproduceapathogen-associatedC3-convertasethatcleavesC3intotheopsoninC3bandtheinflammatoryanaphylotoxinC3a.
Theproteolyticcascadeculminatesinpathogenlysisviadepositionandpolymeri-zationofpore-formingmembraneattackcomplexes(MACs)onopsonizedinvaders[142].
E.
histolyticacanactivateboththealternativebranchandtheserum-antibody-dependentclassicalbranchofthecom-plementcascade[143,144].
AneutralE.
histolyticacysteineproteasecleavesC3toanactiveisoformofC3b[144].
Introphozoitesthataresusceptibletocomplement,C3b,acleavageproductofamebicCP,successfullyrecruitstermi-naleffectorsofthecascaderesultinginMACformationandparasitelysis[145].
Trophozoitesisolatedfromasymptom-aticcasesarereadilylysedinacomplement-dependentmanner,butpathogenictrophozoitesfrompatientswithamebicliverabscessorinvasivecolitisareresistanttocomplement-mediatedkilling[146].
ThesestudiessuggestthatcomplementisanimportantbarrieragainstinvasionbyE.
histolytica,presumablycontributingtothelowincidenceofextraintestinaldisease.
Furthermore,amebicresistancetocomplement-mediatedkillingrepresentsanadaptationuniquetotrophozoitesthatcauseextraintestinaldisease.
Hostcellspreventtheirowncomplement-mediatedlysisbyexpressingsurfaceCD59.
ExpressionofCD59inhibitsopso-nizationandMACformationonhostcells[147].
TheGal/GalNaclectinofE.
histolyticaisamultisubunitGPI-anchoredcomplexessentialforparasiterecognitionofhostsurfaces.
Bragaetal.
showedthatthislectincontainsaCD59-likeregionthatinhibitsMACformationandprotectstheparasitefromcomplement-mediatedlysis[148].
Inagreementwiththeseresults,globalinhibitionofGPIanchorformationleavespreviouslyresistantE.
histolyticatrophozoitessusceptibletocomplement-mediatedlysis[149].
OtherCD59-likeamebicproteinsmayalsocontributetoresistance.
Venturaetal.
recentlyidentifiedanovel21kDaamebicsurfaceproteinthatreactswithhumananti-CD59[150].
However,itsfunctional-ityasaninhibitorofMACformationanditsmoleculariden-tityhaveyettobeelucidated.
Amebiccysteineproteasesalsoplayaroleincomplementevasion.
ThesurfaceEhCPimplicatedinAPactivationbyC3cleavage[144]alsocleavesandinactivatestheinflammatoryanaphylotoxinsC3aandC5a[110].
ThusCPsappearstoplayopposingrolesinevasionofhostimmunitybyE.
histolytica,ontheonehandactivatingcomplementandontheothersuppressingnumerousinflammatorypathwaysdownstreamFig.
3MacrophagesrecruitedtothesiteofE.
histolyticainfectionareclassicallyactivatedbyinterferongammaandothercytokines.
TheyproduceNOtokillpathogensandsecreteIL-10andIL-8,promotinggutbarrierfunctionandneutrophilrecruitment,respectively.
Thepar-asiticarginasecompetitivelyinhibitsandtheMLIFaltersexpressionofhostnitrousoxidesynthase.
E.
histolyticaCpGDNAactivatesTLR9780SeminImmunopathol(2012)34:771–785ofC3aandC5a.
Athirdmechanismofcomplementresistancemayinvolveenrichmentoflypophosphoglycan(LPG)intheouterleafletoftheE.
hystolyticaplasmamembrane.
WhileLPGisproducedinthepathogenicE.
histolytica,significantlevelsarenotobservedinthenon-pathogenicstrainE.
dispar[151].
LPGprotectsLeishmaniafromcomplement-mediatedlysisbyimpedingthedepositionofproteolyticcomplexesandpreventingMACformation[152].
E.
histolyticatrophozoitesmayemployasimilarstrategy.
ALAisapproximatelyfivetimesmoreprevalentinmenthaninwomen,suggestingasexualdimorphisminsusceptibil-itytoextraintestinalamebiasis[153].
Differencesinthecyto-pathogeniceffectsofcomplementonE.
histolyticabetweenthetwosexeshavebeenimplicated[9].
Nonimmunefemaleserumkillstrophozoitesmoreeffectivelythandoesnonimmunemaleserum[154].
However,itisunlikelythatcomplementissolelyresponsibleforthedifferenceinsusceptibilitytoextraintestinaldisease.
Interestingly,thesexualdimorphismobservedinhumanswascorroboratedinmousestudies[134].
FemaleB6miceweremoreeffectivethanmalesatclearinginvasivetroph-ozoitesfromtheliverinaSCIDmousemodelofALA[141].
ClearancewasassociatedwitharapidNKTcellresponseandhighIFN-γproduction[141].
Malemiceclearedinfectionslesseffectively,IFN-γproductionwaslessrobust,andsignificantIL-4productionwasobserved.
ConclusionsGenerationsofresearchhaveprovidedatremendousbodyofknowledgeabouthostimmunitytoamebiasis.
Recentworkhashighlightedtheimportanceoftheparasitegenomeandtran-scriptomeinprogressiontoavirulentphenotype.
Significantprogresshasalsobeenmadeinvaccineanddrugdevelopmentandwenowhaveabetterunderstandingofenvironmentalfactorsthatcontributetoamebiasis.
ThecomplexityoftheinteractionbetweenE.
histolyticatrophozoitesandhost-innateimmunitycontinuestomakeidentificationoffactorsthatinflu-encesusceptibilitytoinfectionafascinatingchallenge.
Effec-tiveapproacheshaveunitedpowerfultechniquesinpopulationgenetics,immunology,andepidemiologytoprobethefullcomplexityoftherelationship.
Usingworkinhumanpopula-tionstoinformmouseandinvitrostudiesofamebiasishasbeenparticularlyeffective,allowingustofocusonhostandparasitefactorsproventoeffectimmunityinhumans.
References1.
HaqueR,MondalD,KirkpatrickBDetal(2003)EpidemiologicandclinicalcharacteristicsofacutediarrheawithemphasisonEntamoebahistolyticainfectionsinpreschoolchildreninanurbanslumofDhaka,Bangladesh.
AmJTropMedHyg69(4):398–4052.
Espinosa-CantellanoM,Martínez-PalomoA(2000)Pathogenesisofintestinalamebiasis:frommoleculestodisease.
ClinMicrobiolRev13(2):318–3313.
BlackRE,AllenLH,BhuttaZAetal(2008)Maternalandchildundernutrition:globalandregionalexposuresandhealthconse-quences.
Lancet371(9608):243–2604.
PetriWAJr,ChaudhryO,HaqueR,HouptE(2006)Adherence-blockingvaccineforamebiasis.
ArchivesofMedicalResearch37(2):287–2905.
HaqueR,AliIM,SackRB(2001)AmebiasisandmucosalIgAantibodyagainsttheEntamoebahistolyticaadherencelectininBangladeshichildren.
JInfectDis183(12):1787–17936.
AbdAllaMD,WolfR,WhiteGL(2012)Efficacyofagal-lectinsubunitvaccineagainstexperimentalEntamoebahistolyticainfec-tionandcolitisinbaboons(Papiosp.
).
Vaccine30(20):3068–30757.
GuoX,BarrosoL,BeckerSMetal(2009)ProtectionagainstintestinalamebiasisbyarecombinantvaccineistransferablebyTcellsandmediatedbygammainterferon.
InfectImmun77(9):3909–39188.
GuoX,BarrosoL,LyerlyDM,PetriWA,HouptER(2011)CD4+andCD8+Tcell-andIL-17-mediatedprotectionagainstEnt-amoebahistolyticainducedbyarecombinantvaccine.
Vaccine29(4):772–7779.
MortimerL,ChadeeK(2010)TheimmunopathogenesisofEnt-amoebahistolytica.
ExpParasitol126(3):366–38010.
GuoX,HouptE,PetriWAJr(2007)Crosstalkattheinitialencounter:interplaybetweenhostdefenseandamebasurvivalstrategies.
CurrOpinImmunol19(4):376–38411.
BillerL,DavisPH,TillackMetal(2010)DifferencesinthetranscriptomesignaturesoftwogeneticallyrelatedEntamoebahistolyticacelllinesderivedfromthesameisolatewithdifferentpathogenicproperties.
BMCGenomics11:6312.
MirelmanD,AnbarM,BrachaR(2008)TrophozoitesofEnt-amoebahistolyticaepigeneticallysilencedinseveralgenesarevirulence-attenuated.
Parasite15(3):266–27413.
BrachaR,NuchamowitzY,AnbarM,MirelmanD(2006)Tran-scriptionalsilencingofmultiplegenesintrophozoitesofEnt-amoebahistolytica.
PLoSPathog2(5):e4814.
QueX,ReedSL(2000)Cysteineproteinasesandthepathogen-esisofamebiasis.
ClinMicrobiolRev13(2):196–20615.
CostaCA,NunesC,FerreiraAJ,GomesMA,CaliariMV(2010)EntamoebahistolyticaandE.
dispartrophozoitesintheliverofhamsters:invivobindingofantibodiesandcomplement.
ParasitVectors3:2316.
AliIKM,ClarkCG,PetriWA(2008)Molecularepidemiologyofamebiasis.
InfectGenetEvol8(5):698–70717.
AliIKM,Solaymani-MohammadiS,AkhterJetal(2008)TissueinvasionbyEntamoebahistolytica:evidenceofgeneticselectionand/orDNAreorganizationeventsinorgantropism.
PLoSNeglTropDis2(4):e21918.
GilchristCA,HouptE,TrapaidzeNetal(2006)ImpactofintestinalcolonizationandinvasionontheEntamoebahistolyticatranscriptome.
MolBiochemParasitol147(2):163–17619.
MorenoH,LinfordAS,GilchristCA,PetriWA(2010)Phospholipid-bindingproteinEhC2Amediatescalcium-dependenttranslocationoftranscriptionfactorURE3-BPtotheplasmamem-braneofEntamoebahistolytica.
EukaryotCell9(5):695–70420.
GilchristCA,MooreES,ZhangYetal(2010)RegulationofvirulenceofEntamoebahistolyticabytheURE3-BPtranscriptionfactor.
mBio1(1):e00057-1021.
TengkuSA,NorhayatiM(2011)Publichealthandclinicalim-portanceofamoebiasisinMalaysia:areview.
TropBiomed28(2):194–22222.
SinghA,HouptE,PetriWA(2009)Rapiddiagnosisofintestinalparasiticprotozoa,withafocusonEntamoebahistolytica.
Inter-discipPerspectInfectDis2009:547090SeminImmunopathol(2012)34:771–78578123.
BlackRE,BrownKH,BeckerS(1984)Malnutritionisadeter-miningfactorindiarrhealduration,butnotincidence,amongyoungchildreninalongitudinalstudyinruralBangladesh.
AmJClinNutr39(1):87–9424.
YoonPW,BlackRE,MoultonLH,BeckerS(1997)Theeffectofmalnutritionontheriskofdiarrhealandrespiratorymortalityinchildren<2yofageinCebu,Philippines.
AmJClinNutr65(4):1070–107725.
MondalD,HaqueR,SackRB,KirkpatrickBD,PetriWA(2009)Attributionofmalnutritiontocause-specificdiarrhealillness:evidencefromaprospectivestudyofpreschoolchildreninMir-pur,Dhaka,Bangladesh.
AmJTropMedHyg80(5):824–82626.
MondalD,MinakJ,AlamMetal(2012)Contributionofentericinfection,alteredintestinalbarrierfunction,andmaternalmalnu-tritiontoinfantmalnutritioninBangladesh.
ClinInfectDis54(2):185–19227.
ChandraRK(1992)Protein-energymalnutritionandimmunolog-icalresponses.
JNutr122(3):597–60028.
ChristadossP,TalalN,LindstromJ,FernandesG(1984)Sup-pressionofcellularandhumoralimmunitytoT-dependentanti-gensbycalorierestriction.
CellImmunol88(1):1–829.
GuerrantRL,SchorlingJB,McAuliffeJF,DeSouzaMA(1992)Diarrheaasacauseandaneffectofmalnutrition:diarrheapre-ventscatch-upgrowthandmalnutritionincreasesdiarrheafre-quencyandduration.
AmJTropMedHyg47(1Suppl):28–3530.
GuerrantRL,OriáRB,MooreSR,OriáMO,LimaAA(2008)Malnutritionasanentericinfectiousdiseasewithlong-termeffectsonchilddevelopment.
NutrRev66(9):487–50531.
SavinoW,DardenneM(2010)Nutritionalimbalancesandinfec-tionsaffectthethymus:consequencesonT-cell-mediatedim-muneresponses.
ProcNutrSoc69(04):636–64332.
AabyP,MarxC,TrautnerSetal(2002)Thymussizeatbirthisassociatedwithinfantmortality:acommunitystudyfromGuinea-Bissau.
ActaPaediatrica91(6):698–70333.
SchaibleUE,KaufmannSHE(2007)Malnutritionandinfection:complexmechanismsandglobalimpacts.
PLoSMed4(5):e11534.
MondalD,PetriWAJr,SackRB,KirkpatrickBD,HaqueR(2006)Entamoebahistolytica-associateddiarrhealillnessisneg-ativelyassociatedwiththegrowthofpreschoolchildren:evi-dencefromaprospectivestudy.
TransactionsoftheRoyalSocietyofTropicalMedicineandHygiene100(11):1032–103835.
HaqueR,MondalD,KarimAetal(2009)Prospectivecase–controlstudyoftheassociationbetweencommonentericprotozoalpara-sitesanddiarrheainBangladesh.
ClinInfectDis48(9):1191–119736.
DuggalP,HaqueR,RoySetal(2004)InfluenceofhumanleukocyteantigenclassIIallelesonsusceptibilitytoEntamoebahistolyticainfectioninBangladeshichildren.
JInfectDis189(3):520–52637.
DuggalP,GuoX,HaqueRetal(2011)AmutationintheleptinreceptorisassociatedwithEntamoebahistolyticainfectioninchildren.
JClinInvest121(3):1191–119838.
LocksleyRM,KilleenN,LenardoMJ(2001)TheTNFandTNFreceptorsuperfamilies:integratingmammalianbiology.
Cell104(4):487–50139.
SéguinR,MannBJ,KellerK,ChadeeK(1995)Identificationofthegalactose-adherencelectinepitopesofEntamoebahistolyticathatstimulatetumornecrosisfactor-alphaproductionbymacro-phages.
ProcNatlAcadSciUSA92(26):12175–1217940.
BlazquezS,ZimmerC,GuigonGetal(2006)HumantumornecrosisfactorisachemoattractantfortheparasiteEntamoebahistolytica.
InfectImmun74(2):1407–141141.
DenisM,ChadeeK(1989)Cytokineactivationofmurinemacro-phagesforinvitrokillingofEntamoebahistolyticatrophozoites.
InfectImmun57(6):1750–175642.
SéguinR,MannBJ,KellerK,ChadeeK(1997)Thetumornecrosisfactoralpha-stimulatingregionofgalactose-inhibitablelectinofEntamoebahistolyticaactivatesgammainterferon-primedmacrophagesforamebicidalactivitymediatedbynitricoxide.
InfectImmun65(7):2522–252743.
ZhangZ,MahajanS,ZhangX,StanleySL(2003)TumornecrosisfactoralphaisakeymediatorofgutinflammationseeninamebiccolitisinhumanintestineintheSCIDmouse–humanintestinalxenograftmodelofdisease.
InfectImmun71(9):5355–535944.
PetersonKM,ShuJ,DuggalPetal(2010)AssociationbetweenTNF-αandEntamoebahistolyticadiarrhea.
AmJTropMedHyg82(4):620–62545.
WangW,KellerK,ChadeeK(1992)ModulationoftumornecrosisfactorproductionbymacrophagesinEntamoebahisto-lyticainfection.
InfectImmun60(8):3169–317446.
HaqueR,MondalD,ShuJetal(2007)Correlationofinterferon-Γproductionbyperipheralbloodmononuclearcellswithchild-hoodmalnutritionandsusceptibilitytoamebiasis.
AmJTropMedHyg76(2):340–34447.
HamanoS,AsgharpourA,StroupSEetal(2006)ResistanceofC57BL/6micetoamoebiasisismediatedbynonhemopoieticcellsbutrequireshemopoieticIL-10production.
JImmunol177(2):1208–121348.
VinoloMAR,FockRA,CrismaARetal(2008)Protein-energymalnutritionmodifiestheproductionofinterleukin-10inre-sponsetolipopolysaccharide(LPS)inamurinemodel.
JNutrSciVitaminol54(5):371–37749.
FaggioniR,FeingoldKR,GrunfeldC(2001)Leptinregulationoftheimmuneresponseandtheimmunodeficiencyofmalnutrition.
FASEBJ15(14):2565–257150.
PalacioA,LopezM,Perez-BravoF,MonkebergF,SchlesingerL(2002)Leptinlevelsareassociatedwithimmuneresponseinmalnourishedinfants.
JCEM87(7):3040–304651.
MooreSE,MorganG,CollinsonACetal(2002)Leptin,malnu-trition,andimmuneresponseinruralGambianchildren.
ArchDisChild87(3):192–19752.
GuoX,RobertsMR,BeckerSMetal(2011)LeptinsignalinginintestinalepitheliummediatesresistancetoentericinfectionbyEntamoebahistolytica.
MucosalImmunol4(3):294–30353.
MooreSI,HuffnagleGB,ChenG-H,WhiteES,MancusoP(2003)LeptinmodulatesneutrophilphagocytosisofKlebsiellapneumoniae.
InfectImmun71(7):4182–418554.
BrunoA,ConusS,SchmidI,SimonH-U(2005)Apoptoticpathwaysareinhibitedbyleptinreceptoractivationinneutro-phils.
JImmunol174(12):8090–809655.
BjrbkC,El-HaschimiK,FrantzJD,FlierJS(1999)TheroleofSOCS-3inleptinsignalingandleptinresistance.
JBiolChem274(42):30059–3006556.
KiguchiN,MaedaT,KobayashiY,FukazawaY,KishiokaS(2009)LeptinenhancesCC-chemokineligandexpressionincul-turedmurinemacrophage.
BiochemBiophysResCommun384(3):311–31557.
GruenML,HaoM,PistonDW,HastyAH(2007)Leptinrequirescanonicalmigratorysignalingpathwaysforinductionofmono-cyteandmacrophagechemotaxis.
AmJPhysiolCellPhysiol293(5):C1481–C148858.
LeeF-YJ,LiY,YangEKetal(1999)Phenotypicabnormalitiesinmacrophagesfromleptin-deficient,obesemice.
AmJPhysiolCellPhysiol276(2):C386–C39459.
LoffredaS,YangSQ,LinHZetal(1998)Leptinregulatesproinflammatoryimmuneresponses.
FASEBJ12(1):57–6560.
Santos-AlvarezJ,GobernaR,Sánchez-MargaletV(1999)Hu-manleptinstimulatesproliferationandactivationofhumancir-culatingmonocytes.
CellImmunol194(1):6–1161.
Sánchez-PozoC,Rodriguez-BaoJ,Domínguez-CastellanoAetal(2003)Leptinstimulatestheoxidativeburstincontrolmono-cytesbutattenuatestheoxidativeburstinmonocytesfromHIV-infectedpatients.
ClinExpImmunol134(3):464–469782SeminImmunopathol(2012)34:771–78562.
GabayC,DreyerMG,PellegrinelliN,ChicheporticheR,MeierCA(2001)Leptindirectlyinducesthesecretionofinterleukin1receptorantagonistinhumanmonocytes.
JCEM86(2):783–79163.
MeierCA,ChicheporticheR,DreyerM,DayerJ-M(2003)IP-10,butnotRANTES,isupregulatedbyleptininmonocyticcells.
Cytokine21(1):43–4764.
BatraA,OkurB,GlaubenRetal(2010)Leptin:acriticalregulatorofCD4+T-cellpolarizationinvitroandinvivo.
Endo-crinology151(1):56–6265.
PadidarS,FarquharsonAJ,WilliamsLMetal(2011)Leptinup–regulatespro–inflammatorycytokinesindiscretecellswithinmousecolon.
JCellPhysiol226(8):2123–213066.
SitaramanS,LiuX,CharrierL,etal(2004)Colonicleptin:sourceofanovelpro-inflammatorycytokineinvolvedininflam-matoryboweldisease.
FASEBJ.
Availableat:http://www.
fasebj.
org/content/early/2004/03/31/fj.
03-0422fje.
Accessed15March201267.
ElKasmiKC,HolstJ,CoffreMetal(2006)GeneralnatureoftheSTAT3-activatedanti-inflammatoryresponse.
JImmunol177(11):7880–788868.
HruzP,DannSM,EckmannL(2010)STAT3anditsactivatorsinintestinaldefenseandmucosalhomeostasis.
CurrOpinGastro-enterol26(2):109–11569.
GoveME,RhodesDH,PiniMetal(2009)Roleofleptinreceptor-inducedSTAT3signalinginmodulationofintestinalandhepaticinflammationinmice.
JLeukocBiol85(3):491–49670.
ChenC,ChangY-C,LiuC-Letal(2007)LeptinInducesprolif-erationandanti-apoptosisinhumanhepatocarcinomacellsbyup-regulatingcyclinD1anddown-regulatingBaxviaajanuskinase2-linkedpathway.
EndocrRelatCancer14(2):513–52971.
OgunwobiO,BealesI(2007)Theanti-apoptoticandgrowthstimulatoryactionsofleptininhumancoloncancercellsinvolvesactivationofJNKmitogenactivatedproteinkinase,JAK2andPI3kinase/Akt.
IntJColorDis22(4):401–40972.
SukhotnikI,HelouH,LurieMetal(2007)Theeffectofleptinonintestinalrecoveryfollowingischemia-reperfusioninjuryinarat.
PediatrSurgInt23(5):473–47873.
SukhotnikI,VadaszZ,CoranAetal(2006)Effectofleptinonintestinalre-growthfollowingmassivesmallbowelresectioninrat.
PediatrSurgInt22(1):9–1574.
MattioliB,StrafaceE,QuarantaMG,GiordaniL,VioraM(2005)LeptinpromotesdifferentiationandsurvivalofhumandendriticcellsandlicensesthemforTh1priming.
JImmunol174(11):6820–682875.
LamQLK,LiuS,CaoX,LuL(2006)Involvementofleptinsignalinginthesurvivalandmaturationofbonemarrow–deriveddendriticcells.
EurJImmunol36(12):3118–313076.
MattioliB,GiordaniL,QuarantaMG,VioraM(2009)Leptinexertsananti-apoptoticeffectonhumandendriticcellsviathePI3K-Aktsignalingpathway.
FEBSLett583(7):1102–110677.
MarieCS,VerkerkeHP,PaulSN,MackeyAJ,PetriWA(2012).
LeptinprotectshostcellsfromEntamoebahistolyticacytotoxic-itybyaSTAT-3dependentmechanism.
InfectImmun.
Availableat:http://iai.
asm.
org/content/early/2012/02/06/IAI.
06140-11.
Accessed24February201278.
WankeC,ButlerT,IslamM(1988)EpidemiologicandclinicalfeaturesofinvasiveamebiasisinBangladesh:acase–controlcomparisonwithotherdiarrhealdiseasesandpostmortemfind-ings.
AmJTropMedHyg38(2):335–34179.
WallaceJL,VongL,DharmaniP,SrivastavaV,ChadeeK(2011)Muc-2-deficientmicedisplayasex-specific,COX-2-relatedimpair-mentofgastricmucosalrepair.
AmJPathol178(3):1126–113380.
BergstromKSB,Kissoon-SinghV,GibsonDLetal(2010)Muc2protectsagainstlethalinfectiouscolitisbydisassociatingpatho-genicandcommensalbacteriafromthecolonicmucosa.
PLoSPathog6(5):e100090281.
ChadeeK,PetriWA,InnesDJ,RavdinJI(1987)RatandhumancolonicmucinsbindtoandinhibitadherencelectinofEntamoebahistolytica.
JClinInvest80(5):1245–125482.
MoncadaDM,KammanadimintiSJ,ChadeeK(2003)MucinandToll-likereceptorsinhostdefenseagainstintestinalparasites.
TrendsParasitol19(7):305–31183.
LidellME,MoncadaDM,ChadeeK,HanssonGC(2006)Ent-amoebahistolyticacysteineproteasescleavetheMUC2mucininitsC-terminaldomainanddissolvetheprotectivecolonicmucusgel.
ProcNatlAcadSciUSA103(24):9298–930384.
BansalD,AveP,KerneisSetal(2009)Anex-vivohumanintestinalmodeltostudyEntamoebahistolyticapathogenesis.
PLoSNeglTropDis3(11):e55185.
BosHJ,vandeGriendRJ(1977)VirulenceandtoxicityofaxenicEntamoebahistolytica.
Nature265(5592):341–34386.
BhattacharyaA,GhildyalR,PrasadJ,BhattacharyaS,DiamondLS(1992)ModulationofasurfaceantigenofEntamoebahisto-lyticainresponsetobacteria.
InfectImmun60(4):1711–171387.
RaniR,MurthyRS,BhattacharyaSetal(2006)Changesinbacte-rialprofileduringamebiasis:demonstrationofanaerobicbacteriainALApussamples.
AmJTropMedHyg75(5):880–88588.
CrawfordPA,CrowleyJR,SambandamNetal(2009)Regulationofmyocardialketonebodymetabolismbythegutmicrobiotaduringnutrientdeprivation.
ProcNatlAcadSciUSA106:11276–1128189.
JumpertzR,LeDS,TurnbaughPJetal(2011)Energy-balancestudiesrevealassociationsbetweengutmicrobes,caloricload,andnutrientabsorptioninhumans.
AmJClinNutr94(1):58–6590.
KauAL,AhernPP,GriffinNW,GoodmanAL,GordonJI(2011)Humannutrition,thegutmicrobiome,andimmunesystem:envi-sioningthefuture.
Nature474(7351):327–33691.
GuptaSS,MohammedMH,GhoshTSetal(2011)Metagenomeofthegutofamalnourishedchild.
GutPathog3:792.
TovyA,HertzR,Siman-TovRetal(2011)GlucosestarvationboostsEntamoebahistolyticavirulence.
PLoSNeglTropDis5(8):e124793.
Serrano-LunaJ,Gutiérrez-MezaM,Mejía-ZepedaRetal(2010)Effectofphosphatidylcholine-cholesterolliposomesonEntamoe-bahistolyticavirulence.
CanJMicrobiol56(12):987–99594.
LeeJ,ParkSJ,YongTS(2008)EffectofirononadherenceandcytotoxicityofEntamoebahistolyticatoCHOcellmonolayers.
KoreanJParasitol46(1):37–4095.
CoboER,HeC,HirataKetal(2012)Entamoebahistolyticainducesintestinalcathelicidinsbutisresistanttocathelicidin-mediatedkilling.
InfectImmun80(1):143–14996.
GranlundAVB,BeisvagV,TorpSHetal(2011)ActivationofREGfamilyproteinsincolitis.
ScandJGastroenterol46(11):1316–132397.
PetersonKM,GuoX,ElkahlounAGetal(2011)TheexpressionofREG1AandREG1Bisincreasedduringacuteamebiccolitis.
ParasitolInt60(3):296–30098.
MukherjeeS,VaishnavaS,HooperLV(2008)Multi-layeredregulationofintestinalantimicrobialdefense.
CellMolLifeSci65(19):3019–302799.
KollsJK,McCrayPB,ChanYR(2008)Cytokine-mediatedregu-lationofantimicrobialproteins.
NatRevImmunol8(11):829–835100.
KammanadimintiSJ,MannBJ,DutilL,ChadeeK(2004)Reg-ulationofToll-likereceptor-2expressionbytheGal-lectinofEntamoebahistolytica.
FASEBJ18(1):155–157101.
BeckerSM,ChoK-N,GuoXetal(2010)EpithelialcellapoptosisfacilitatesEntamoebahistolyticainfectioninthegut.
AmJPathol176(3):1316–1322102.
EckmannL,ReedSL,SmithJR,KagnoffMF(1995)Entamoebahistolyticatrophozoitesinduceaninflammatorycytokinere-sponsebyculturedhumancellsthroughtheparacrineactionofcytolyticallyreleasedinterleukin-1alpha.
JClinInvest96(3):1269–1279SeminImmunopathol(2012)34:771–785783103.
HouY,MortimerL,ChadeeK(2010)Entamoebahistolyticacysteineproteinase5bindsintegrinoncoloniccellsandstimu-latesNFκB-mediatedpro-inflammatoryresponses.
JBiolChem285(46):35497–35504104.
IvoryCPA,PrystajeckyM,JobinC,ChadeeK(2008)Toll-likereceptor9-dependentmacrophageactivationbyEntamoebahis-tolyticaDNA.
InfectImmun76(1):289–297105.
SeydelKB,LiE,SwansonPE,StanleySL(1997)Humanintes-tinalepithelialcellsproduceproinflammatorycytokinesinre-sponsetoinfectioninaSCIDmouse–humanintestinalxenograftmodelofamebiasis.
InfectImmun65(5):1631–1639106.
KammanadimintiSJ,ChadeeK(2006)SuppressionofNF-κBactivationbyEntamoebahistolyticainintestinalepithelialcellsismediatedbyheatshockprotein27.
JBiolChem281(36):26112–26120107.
SalataRA,AhmedP,RavdinJI(1989)ChemoattractantactivityofEntamoebahistolyticaforhumanpolymorphonuclearneutro-phils.
JParasitol75(4):644–646108.
ChadeeK,MoreauF,MeerovitchE(1987)Entamoebahistoly-tica:chemoattractantactivityforgerbilneutrophilsinvivoandinvitro.
ExpParasitol64(1):12–23109.
YuY,ChadeeK(1997)Entamoebahistolyticastimulatesinter-leukin8fromhumancolonicepithelialcellswithoutparasite–enterocytecontact.
Gastroenterology112(5):1536–1547110.
ReedS,EmberJ,HerdmanDetal(1995)TheextracellularneutralcysteineproteinaseofEntamoebahistolyticadegradesanaphylatoxinsC3aandC5a.
JImmunol155(1):266–274111.
SalataRA,RavdinJI(1986)TheinteractionofhumanneutrophilsandEntamoebahistolyticaincreasescytopathogenicityforlivercellmonolayers.
JInfectDis154(1):19–26112.
GuerrantRL,BrushJ,RavdinJI,SullivanJA,MandellGL(1981)InteractionbetweenEntamoebahistolyticaandhumanpolymorphonuclearneutrophils.
JInfectDis143(1):83–93113.
MontecuccoF,BianchiG,GnerrePetal(2006)Inductionofneutrophilchemotaxisbyleptin.
AnnNYAcadSci1069(1):463–471114.
Caldefie-ChezetF,PoulinA,TridonA,SionB,VassonM-P(2001)Leptin:apotentialregulatorofpolymorphonuclearneu-trophilbactericidalactionJLeukocBiol69(3):414–418115.
NathanC(2006)Neutrophilsandimmunity:challengesandopportunities.
NatRevImmunol6(3):173–182116.
BrinkmannV,ReichardU,GoosmannCetal(2004)Neutrophilextracellulartrapskillbacteria.
Science303(5663):1532–1535117.
Rivero-NavaL,Aguirre-GarcaJ,Shibayama-SalasMetal(2002)Entamoebahistolytica:acutegranulomatousintestinallesionsinnormalandneutrophil-depletedmice.
ExpParasitol101(4):183–192118.
Jarillo-LunaR,Campos-RodrguezR,TsutsumiV(2002)Ent-amoebahistolytica:immunohistochemicalstudyofhepaticam-oebiasisinmouse.
Neutrophilsandnitricoxideaspossiblefactorsofresistance.
ExpParasitol101(1):40–56119.
RigothierM-C,KhunH,TavaresPetal(2002)FateofEntamoe-bahistolyticaduringestablishmentofamoebicliverabscessanalyzedbyquantitativeradioimagingandhistology.
InfectImmun70(6):3208–3215120.
SeydelKB,ZhangT,StanleySL(1997)Neutrophilsplayacriticalroleinearlyresistancetoamebicliverabscessesinseverecombinedimmunodeficientmice.
InfectImmun65(9):3951–3953121.
ChoiM-H,SajedD,PooleLetal(2005)AnunusualsurfaceperoxiredoxinprotectsinvasiveEntamoebahistolyticafromoxi-dantattack.
MolBiochemParasitol143(1):80–89122.
BruchhausI,RichterS,TannichE(1997)Removalofhydrogenperoxidebythe29kDaproteinofEntamoebahistolytica.
Bio-chemJ326(Pt3):785–789123.
Maldonado–BernalC,KirschningCJ,RosensteinYetal(2005)TheinnateimmuneresponsetoEntamoebahistolyticalipopeptidophosphoglycanismediatedbytoll–likereceptors2and4.
ParasiteImmunology27(4):127–137124.
SeydelKB,SmithSJ,StanleySL(2000)Innateimmunitytoamebicliverabscessisdependentongammainterferonandnitricoxideinamurinemodelofdisease.
InfectImmun68(1):400–402125.
Siman-TovR,AnkriS(2003)Nitricoxideinhibitscysteineproteinasesandalcoholdehydrogenase2ofEntamoebahistoly-tica.
ParasitolRes89(2):146–149126.
ElnekaveK,Siman–TovR,AnkriS(2003)ConsumptionofL–argininemediatedbyEntamoebahistolyticaL–arginase(EhArg)inhibitsamoebicidalactivityandnitricoxideproductionbyacti-vatedmacrophages.
ParasiteImmunology25(11–12):597–608127.
WangW,KellerK,ChadeeK(1994)Entamoebahistolyticamodulatesthenitricoxidesynthasegeneandnitricoxideproduc-tionbymacrophagesforcytotoxicityagainstamoebaeandtu-mourcells.
Immunology83(4):601–610128.
LinJY,KellerK,ChadeeK(1993)Entamoebahistolyticapro-teinsmodulatetherespiratoryburstpotentialbymurinemacro-phages.
Immunology78(2):291–297129.
SeguinR,KellerK,ChadeeK(1995)Entamoebahistolyticastimulatestheunstabletranscriptionofc-fosandtumournecrosisfactor-alphamRNAbyproteinkinaseCsignaltransductioninmacrophages.
Immunology86(1):49–57130.
DeyI,KellerK,BelleyA,ChadeeK(2003)Identificationandcharacterizationofacyclooxygenase-likeenzymefromEntamoe-bahistolytica.
ProcNatlAcadSciUSA100(23):13561–13566131.
DeyI,ChadeeK(2008)ProstaglandinE2producedbyEntamoe-bahistolyticabindstoEP4receptorsandstimulatesinterleukin-8productioninhumancoloniccells.
InfectImmun76(11):5158–5163132.
Rico-RosilloG,Díaz-GuerraO,KretschmerRR(1990)[Mono-cytelocomotioninhibitingfactor(MLIF)producedbyE.
histo-lyticainducesanincreaseofcAMPinhumanmonocytes].
ArchInvestMed(Mex)21(Suppl1):245–247133.
KretschmerR,CastroEM,ArellanoJ,PachecoMG(1986)InvitrostudiesontheinteractionofhumanmonocytesandthemonocytelocomotioninhibitoryfactorproducedbyE.
histoly-tica.
ArchInvestMed(Mex)17(Suppl1):243–246134.
LotterH,JacobsT,GaworskiI,TannichE(2006)Sexualdimor-phisminthecontrolofamebicliverabscessinamousemodelofdisease.
InfectImmun74(1):118–124135.
GordonS,MartinezFO(2010)Alternativeactivationofmacro-phages:mechanismandfunctions.
Immunity32(5):593–604136.
CohenNR,GargS,BrennerMB(2009).
Chapter1antigenpresen-tationbyCD1:lipids,Tcells,andNKTcellsinmicrobialimmunity.
In:AdvancesinImmunology.
Volume102.
AcademicPress,pp.
1–94.
Availableat:http://www.
sciencedirect.
com/science/article/pii/S0065277609012012.
Accessed26February2012137.
TsutsumiV,Mena-LopezR,Anaya-VelazquezF,Martinez-PalomoA(1984)Cellularbasesofexperimentalamebicliverabscessformation.
AmJPathol117(1):81–91138.
KimKH,ShinCO,ImK(1993)Naturalkillercellactivityinmiceinfectedwithfree-livingamoebawithreferencetotheirpathogenicity.
TheKoreanJournalofParasitology31(3):239139.
FusakioME,MohammedJP,LaumonnierYetal(2011)C5aregulatesNKTandNKcellfunctionsinsepsis.
JImmunol187(11):5805–5812140.
GodfreyDI,MacDonaldHR,KronenbergM,SmythMJ,KaerLV(2004)NKTcells:what'sinanameNatRevImmunol4(3):231–237141.
LotterH,González-RoldánN,LindnerBetal(2009)NaturalkillerTcellsactivatedbyalipopeptidophosphoglycanfromEnt-amoebahistolyticaarecriticallyimportanttocontrolamebicliverabscess.
PLoSPathog5(5):e1000434142.
DunkelbergerJR,SongW-C(2009)Complementanditsroleininnateandadaptiveimmuneresponses.
CellRes20(1):34–50784SeminImmunopathol(2012)34:771–785143.
Ortiz-OrtizL,CapinR,CapinNR,SepúlvedaB,ZamaconaG(1978)ActivationofthealternativepathwayofcomplementbyEntamoebahistolytica.
ClinExpImmunol34(1):10–18144.
ReedS,KeeneW,McKerrowJ,GigliI(1989)CleavageofC3byaneutralcysteineproteinaseofEntamoebahistolytica.
JImmu-nol143(1):189–195145.
ReedSL,GigliI(1990)Lysisofcomplement-sensitiveEntamoe-bahistolyticabyactivatedterminalcomplementcomponents.
Initiationofcomplementactivationbyanextracellularneutralcysteineproteinase.
JClinInvest86(6):1815–1822146.
ReedS,CurdJ,GigliI,GillinF,BraudeA(1986)ActivationofcomplementbypathogenicandnonpathogenicEntamoebahisto-lytica.
JImmunol136(6):2265–2270147.
Flores-RomoL,TsutsumiV,Estrada-GarcíaTetal(1994)CD59(protectin)molecule,resistancetocomplement,andvirulenceofEntamoebahistolytica.
TransRSocTropMedHyg88(1):116–117148.
BragaLL,NinomiyaH,McCoyJJetal(1992)Inhibitionofthecomplementmembraneattackcomplexbythegalactose-specificadhesionofEntamoebahistolytica.
JClinInvest90(3):1131–1137149.
WeberC,BlazquezS,MarionSetal(2008)Bioinformaticsandfunctionalanalysisofanentamoebahistolyticamannosyltrans-ferasenecessaryforparasitecomplementresistanceandhepaticalinfection.
PLoSNeglTropDis2(2):e165150.
Ventura-JuárezJ,Campos-RodríguezR,Jarillo-LunaRAetal(2008)TrophozoitesofEntamoebahistolyticaexpressaCD59-likemoleculeinhumancolon.
ParasitolRes104(4):821–826151.
BhattacharyaA,AryaR,ClarkCG,AckersJP(2000)Absenceoflipophosphoglycan-likeglycoconjugatesinEntamoebadispar.
Parasitology120(01):31–35152.
SacksD,SherA(2002)Evasionofinnateimmunitybyparasiticprotozoa.
NatImmunol3(11):1041–1047153.
Acuna-SotoR,MaguireJH,WirthDF(2000)Genderdistributioninasymptomaticandinvasiveamebiasis.
AmJGastroenterol95(5):1277–1283154.
SnowM,ChenM,GuoJ,AtkinsonJ,StanleySL(2008)Differ-encesincomplement-mediatedkillingofEntamoebahistolyticabetweenmenandwomen—anexplanationfortheincreasedsusceptibilityofmentoinvasiveamebiasisAmJTropMedHyg78(6):922–923SeminImmunopathol(2012)34:771–785785
Verkerke&WilliamA.
PetriJr.
&ChelseaS.
MarieReceived:17April2012/Accepted:21September2012/Publishedonline:1November2012#Springer-VerlagBerlinHeidelberg2012AbstractEntamoebahistolytica,theprotozoanparasitethatcausesamebicdysentery,greatlycontributestodiseaseburdeninthedevelopingworld.
Effortstoexhaustivelycharacterizethepathogenesisofamebiasishaveincreasedourunderstand-ingofthedynamichost–parasiteinteractionandtheprocessbywhichE.
histolyticatrophozoitestransitionfromgutcom-mensalstoinvadersoftheintestinalepithelium.
Mousemod-elsofdiseasecontinuetobeinstrumentalinthisarea.
Atthesametime,large-scalestudiesinhumanpopulationshaveidentifiedgeneticandenvironmentalfactorsthatinfluencesusceptibilitytoamebiasis.
Nutritionalstatushaslongbeenknowntogloballyinfluenceimmunefunction.
Soitisnotsurprisingthatundernutritionhasemergedasacriticalriskfactor.
AbetterunderstandingofhownutritionalstatusaffectsimmunitytoE.
histolyticawillhavedramaticimplicationsinthedevelopmentofnoveltreatments.
Futureworkshouldcontinuetocharacterizethefascinatinghost–parasitearmsracethatoccursateachstageofinfection.
KeywordsEntamoebahistolytica.
Amebiasis.
Innateimmunity.
UndernutritionIntroductionEntamoebahistolyticaisaprotozoanparasiteendemictomuchofthedevelopingworld.
TheWHOestimatesthat100millionyearlyE.
histolyticainfectionsresultinapproximately100,000deathsfromdysentery,colitis,andextraintestinaldiseasessuchasamebicliverabscess(ALA).
Amebiasisspreadsbyingestionoffoodandwatercontaminatedwithamebiccysts,whichareresistanttothecausticenvironmentofthestomachandpassunimpededintotheintestine.
Excystationandcolonizationoccurinthetermi-nalileum.
E.
histolyticatypicallyestablishesacommensalrelationshipwiththehostandcystsofnonpathogenictroph-ozoitesareexcreted,perpetuatingthelifecycleoftheparasite.
Itisestimatedthat90%ofcasesareasymptomaticwhile10%ofinfectionsprogresstosymptomaticdisease.
Asmallbutsignificantsubpopulationofsymptomaticindividualsdevel-opssevereextraintestinalamebiasis,includingamebicliverabscess[1,2].
IthaslongbeensuspectedbutisnowconfirmedthatundernutritionsignificantlyincreasessusceptibilitytoE.
histolyticainfection.
Undernutritioncontributesto2.
2mil-liondeathsand21%ofdisability-adjustedlifeyearsinchildrenunder5.
Estimatesarethatundernutritionanditsassociateddiseasescontributeto35%ofchilddeathsand11%oftheglobaldiseaseburden[3].
StudiesofamebiasisinhumanpopulationsandmousemodelshaveinformedourknowledgeofhostfactorsthatinfluencesusceptibilitytoE.
histolyticaandparasitefactorsthatmaydirectlyalterviru-lence.
AdaptiveimmunitydevelopsincontinuallyexposedhumanpopulationsandisbelievedtomainlyresultfromproductionofneutralizingantibodiestargetedtotheGal/GalNAcadherencelectin[4].
SecretoryIgAagainstthislectinisassociatedwithprotectioninhumans[5]andhasrecentlybeenshowntocorrelatewithimmunityinexperi-mentallyvaccinatedbaboons[6].
Studiestargetingvaccinedevelopmentinmicesuggestthatprotectiveimmunityismediatedbyaninterferongamma(IFN-γ)-dependentTcellresponseduringinfectionwithE.
histolyticatrophozoites[7,8].
Asvaccinedevelopmentisongoing,thisreviewfocusesontheinnateimmuneresponseduringamebiasis.
WeThisarticleisacontributiontothespecialissueonImmunoparasitology-GuestEditor:MiguelStadeckerH.
P.
Verkerke:W.
A.
PetriJr.
(*):C.
S.
MarieDivisionofInfectiousDiseasesandInternationalHealth,UniversityofVirginiaHealthSystem,MR6buildingRoom1107,29815thSTSW,Charlottesville,VA22903,USAe-mail:wap3g@virginia.
eduSeminImmunopathol(2012)34:771–785DOI10.
1007/s00281-012-0349-1emphasizetheimportanceofenvironmentalfactorsthatalterhostresistancetoamebicdisease,inparticulartheemergingroleofhostnutritionalstatus.
WealsoexplorerecentworkthathasshedlightonbothhostandparasitegeneticfactorsimportantinthepathogenesisofE.
histolyticainfection.
ParasiteKnownamebicvirulencefactorsincludetheGal/GalNAcadherencelectin,cysteineproteases(CPs),arginase,ameba-pores,alcoholdehydrogenase,peroxiredoxin,cyclooxyge-nase2,andlipopeptidophosphoglycan(LPPG)[9,10].
StudiesofE.
histolyticavirulencecontinuetouncovernewfactorsinvolvedinpathogenesisandinteractionwiththehostinnateimmunesystemandmorearelikelytoemergefromfurtheranalysisoftheparasitegenome[11].
Charac-terizedvirulencefactorsofE.
histolyticaareoftendown-regulatedinnonpathogenicisolates[12,13]orabsentinthenonpathogenicamebicspeciesEntamoebadisparandEnt-amoebamoshkovski[14–16]RecentworkinE.
histolyticagenomicssupportsarolefortheparasitegenotypeandgeneticrecombinationintheprogressionofamebiasis.
Alietal.
havefoundthattroph-ozoitesisolatedfromthegutandliverofpatientswithbothintestinalandextraintestinaldiseasearegeneticallydistinctfromparasitesinthestool[17].
Oneexplanationisthatgeneticallydistinctsubpopulationsofvirulenttrophozoitesexistinthegut.
Alternatively,progressionofamebicdiseasemaycorrespondtogeneticreorganizationeventssuchasrecombinationthatpromoteaninvasivephenotype.
Howev-er,thehostandparasiteeffectorsdrivingtheseeventsateachstageofinfectionremainpoorlydefined.
Developingmorepowerfultoolstoperformwithin-patientcomparisonsofamebicvirulenceatdifferentstagesofinfectionwillgreatlyincreaseourunderstandingofthisprocess.
ItalsoremainsunclearwhetherregionalgenomicdifferencesinE.
histolyticastrainsaffectpathogenicity.
Gilchristetal.
compellinglydemonstratedthatexpressionofvirulencegenesisregulatedatthemRNAlevelinthefirstgenome-wideanalysisoftheE.
histolyticatranscriptomein2006.
ThisstudydemonstratedthatinvasivetrophozoitesinmicesignificantlyalteredmRNAexpressionforgeneswithdiverserolesinmetabolismandvirulence[18].
Workonthecalcium-regulatedtranscriptionfactorURE3BPalsosup-portsacrucialrolefortranscriptionalregulationinamebicvirulence.
URE3BPwasoriginallyidentifiedasaregulatoroftheGal/GalNAcadherencelectinandferredoxin.
Laterworkidentifiedacalcium-dependentphospholipid-associatedpeptide,ehC2A,whichseques-tersURE3BPtotheplasmamembraneinhighcalcium[19].
AcomprehensivestudyoftheroleofURE3BPinamebicvirulencehasrevealedthattrophozoitesexpressingadominantpositiveformofURE3BParemorepathogenic,formingdramaticallylargerlesionsintheliversofchallengedgerbils[20].
ThepotentcytotoxiceffectthatE.
histolyticaexertsonhostcellsisevidencethattheparasitepossessesauniquearmamentariumofvirulencefactors.
However,muchworkremainstobedonetodefineparasitefactorsandhowtheyinteractwiththehostateachstageofdisease.
Furtherclarifyingthemechanismsbywhichtrophozoitestransitionfromintestinalcolonizerstoinvasivepathogenshaspoten-tialforfutureanti-parasiticdrugdevelopment.
EnvironmentDiarrhealdiseaseslikeamebiasisareendemictothede-velopingworldwhereanumberofenvironmentalfactorsincludinglimitedaccesstocleanfoodandwater,andexposuretootherenteropathogenscontributetoincreasedriskofinfection[1,3,16,21–23].
Wearenowinthe14thyearoftrackingthemedicalhistoryofacohortofchil-drenlivinginMirpur,anurbanslumofDhaka,Bangla-deshwhereE.
histolyticainfectionisoneofthemajorcausesofdiarrhealillness.
SomeofthemajorfindingsfromthispopulationaresummarizedinTable1.
Apicturehasbeguntoemergeinwhichinfantundernutritionandstuntingpredisposesyoungchildrentoamebiasis,whichinturnexacerbatespreexistingnutritionalimbalancebyalteringgutfunction.
Undernutritionincreasestheriskofdiarrheaanditsas-sociatedmortality[24].
UndernourishedchildrenhavehigherratesofinfectionbyprotozoanparasitesparticularlyE.
histolyticaandCryptosporidiumrelativetowell-nourishedcontrols[25].
ArecentstudyofinfantsfromMirpurinthefirstyearoflifebyMondaletal.
showedthatundernutritionandstuntingat12monthsofageissignifi-cantlyassociatedwithnutritionalstatusatbirth,durationandseverityofdiarrhealepisodes,anddiminishedintestinalbarrierfunction[26].
Whilethisassociationisquiteclear,thecomplexetiologyisnotyetfullyunderstood.
Proteinenergymalnutritionisthemostcommoncauseofhumansecondaryimmunodeficiency,whichisparticularlyproblematicfordiarrhealdiseasesthatfurtherdiminishnu-trientuptakefromthegastrointestinaltract[27–30].
Acutestarvationinitiatesenergy-savingmechanisms,shuntingpowerawayfromimmunefunction.
Chronicnutritionaldeprivationleadstosevereimmunedeficitsexemplifiedbythedramaticthymicatrophyobservedincadaversofunder-nourishedindividuals[31,32].
Complementdeficits,re-ducedIgAproductionandspecificity,alteredgutbarrierfunctionduetorestructuredmicrovilli,impairedphagocyticcapacity,anddiminishedoxidativebursthaveallbeende-scribedasconsequencesofenergydeficitintheimmune772SeminImmunopathol(2012)34:771–785Table1SummaryofstudiesfromMirpuramebiasisbirthcohortAuthorDateStudyMajorfindingsHaqueetal.
2001MeasuredstoolIgAanti-lectinandassociationwithincidenceofEntamoebahistolyticadiarrheaMucousalIgAantibodiesraisedagainsttheamebicGalNAc-lecticareprotectiveagainstE.
histolytica.
Corroboratedin2006Haqueetal.
2002Anti-lecticserumIgGIsolatedtrophozoitesweregeneticallydiverseAnti-lecticfecalIgALackofserumIgGagainsttheGalNAclecticlinkedtoresistancePCRanalysisbyisolatedtrophozoites86%reductioninnewinfectionof12monthsinchildrenwhodevelopedIgAanti-lectinHaqueetal.
2003DiagnostictoolstomeasurecausesofdiarrhealepisodesWell-nourishedchildrenhadfewerepisodesofdiarrhealoverallMostfrequentcausesofdiarrheaincludedenterotoxigenicEscherichiacoli,Shigella,Rotavirus,Giardialamblia,Cryptosporidiumparvum,andE.
histolyticaE.
histolyticacontributedtooverallmorbidityfromdiarrhealillnessDuggaletal.
2004GenotypeleukocyteantigenclassIIallelesChildrenwithDQB1*0601/DRB1*1501haplotypewere10.
1timesmoreresistanttoE.
histolyticaandlesslikelytobesero-positiveforanti-lectinIgGHLAclassII-restrictedimmuneresponsesprotectiveagainstE.
histolyticainfectionTarletonetal.
2006VerbalandnonverbaltestforcognitiveabilityCognitivescorenegativelyassociatedwithstuntinginschool-agechildrenE.
histolyticadiarrheawasnotindependentlyassociatedwithcognitivedeficitswhenthestudywascontrolwithsocialfactorsMondaletal.
2006WeightforageZ-scoreE.
histolytica-associateddiarrhealillnesswasnegativelyassociatedwithgrowthofpreschoolchildrenasmeasuredbyWHOWAZandHAZHeigthforageZ-scoreCryptosporidiumandGiardiadiarrhealepisodesnotassociatedwithgrowthStoolantigendiagnosticsforCryptosporidiumandE.
histolyticaMicroscopicdiagnosicsforGiardiaHaqueetal.
2007IFN-γproductionbyamebicantigenstimulatedPBMCsAbove-averagesecretionofIFN-γbyPBMCsassociatedwithlongersurvivalwithoutE.
histolyticadiarrhealepisodesAdjustedforstunting,theincreasedriskremainedmildlysignificantConcludedthatIFN-γproductionlinkedtonutritionalstatusisprotectiveMondaletal.
2009AnalyzedassociationbetweenE.
histolyticadiarrheaandundaernutritionEnterotoxigenicE.
coli,Cryptosporidium,andE.
histolyticainfectionmorecommoninmalnourishedchildrenMalnutritioncontributestosusceptibilityinasubsetofentericinfectionsPetersonetal.
2010TNF-αproductionbyamebicantigenstimulatedPBMCsHighlevelofTNF-αproductionassociatedwithincreaseriskofthefirstandrecurrentE.
histolyticadiarrhealepisodesMicroarrayanalysisofwholebloodandcolonbiopsysamplesfrompatientsinacuteandconvalescentstagesofamebiasisSymptomaticinfectionwasassociatedwithsignificantlyhigherproductionofTNF-αproteinandmildlyelevatedTNF-αmessagelevelsDuggaletal.
2011MeasuredgeneticvariantsinleptinandtheleptinreceptorandinvestigatedtheirassociationwithamebiasisChildernhomozygousforaSNPencodingasingleaminoacidsubstitutionintheleptinreceptor(Q223R)werefoundtobenearlyfourtimesmorelikelytohaveaninfectioncomparedwiththosehomozygousforthewild-typealleleAdultALApatientswerelesslikelytocarrytheprotectiveglutaminealleleMicecarryingtheprotectivealleleweresignificantlymoreresistanttoexperimentalamebiasisPetersonetal.
2011MicroarrayanalysisofintestinalbiopsiesinacuteandconvalescentamebiasisREG1AandREG1BwerehighlyupregulatedinhumanbiopsiesfrompatientswithacuteversusconvalescentamebiasisRT-qPCRforREG1A/1BResultconfirmedbyRT-qPCRChallengedREG1knockoutmicewithE.
histolyticatrophozoitesREG1knockoutmiceweresignificantlymoresusceptibletoexperimentalamebiasisSeminImmunopathol(2012)34:771–785773system(reviewedin[33]).
Globalsuppressionanddysregu-lationofimmunefunctionassociatedwithacuteandchronicundernutritionmaybethemostimportantindependentpre-dictorofE.
histolyticadiarrheainthefirstyearoflife.
Reciprocally,E.
histolyticadiarrhealepisodesincreasetheriskofbecomingundernourished[23,26,34,35].
Chil-drenwithE.
histolytica-associateddiarrheaarenearlythreetimesmorelikelytobeclinicallyundernourishedandareatdramaticallyincreasedriskofstunting[34].
Childhoodundernutritionanddiarrheaareinextricablylinkedwithprofoundconsequencesfortreatmentout-comesinthedevelopingworld.
Intestinalinflammationresultingfromrepeatedexposuretoentericpathogensleadstomalabsorptionofcriticalnutrients,whilesecond-aryimmunodeficiencyrelatedtocompromisednutritionalstatusmakeschildrenunder5particularlyvulnerabletothedevastatingcycleofundernutrition,disease,andpov-erty.
Tobreakthiscycle,aholisticapproachisneeded.
TreatmentstrategiestotargetE.
histolyticadiarrheainthecontextofanundernourishedpatientmustbedesigned.
Morebroadly,nutritionalsupplementationshouldbeenrichedtoprovideessentialnutrientsaffectedbyrepeat-edexposuretoenteropathogensandsuchsupplementscouldbecomearoutinepartoftreatment.
Finally,suc-cessfulvaccinesmayutilizeadjuvantsthatstimulatein-natefactorsdeficientinundernourishedindividuals.
Whiletherapeuticandnutritionalinterventionsarecritical,humangeneticfactorsthataffectinnateresistancetoE.
histolyticainfectionfurthercomplicatetheinteractionbe-tweennutritionalstatusandparasiticdisease.
HostNaturalimmunityinhumansMountingevidencesuggeststhathostgeneticfactorsinflu-encefrequencyandseverityofE.
histolyticadiarrhealepisodes.
TheHLAclassIIalleleDQB1*0601andthehaplotypeDQB1*0601/DRB1*1501areassociatedwithdrasticallydecreasedratesofE.
histolyticainfection[36].
Morerecently,a12-yearstudyoftheMirpurcohorthasshownthatasingleaminoacidreplacement(Q223R)intheextracellulardomainoftheleptinreceptorencodedbyaSNPisassociatedwithanearlyfourfoldincreaseinsuscep-tibilitytoinfectionbyE.
histolyticaaswellasanoveralldecreaseintimetoinfection.
TheeffectsofthemutationwereconfirmedinapopulationofadultmaleALApatients,wherehomozygosityforthealleleencodingarginineatposition223wasassociatedwithtwofoldincreasedsuscep-tibilitytoextraintestinaldisease.
Theseobservationshigh-lighttheimportanceofleptinsignalingandpointtoaspecificmutationthatincreasessusceptibilitytoE.
histoly-tica,decreasestimetoinfection,andincreasesthelikelihoodofdevelopingextraintestinalamebiasis[37].
Itisclearthatthehostgeneticbackgroundinfluencessusceptibilitytoamebiasis.
However,environmentalfactorscomplicatethepicturebysimultaneouslyaffectingthephe-notype.
Itisimportanttodelineateprimaryresistancedeter-minedbygeneticsandsecondaryresistancedeterminedbynutritionalstatusandotherenvironmentalfactors.
Itiscrit-icaltoidentifymorehostgeneticfactorsthataffectE.
histolyticainfection.
Thesestudieswillnotonlyincreaseourunderstandingofthepathogenesisofamebiasisbutalsoaidintargetingtherapiestoat-riskpopulations.
CytokinesTNF-αTNF-αisacytokinewithdiverserolesinimmunity.
Dependingonthefactorsrecruitedtoligand-boundTNFR1/2,TNF-αcanstimulategrowthanddifferentiationthroughMAPK,survivalandinflammationthroughNFκB,orcelldeaththroughcaspaseactivation[38].
TheamebiclectininducesTNF-αproductionbymacrophages[39]andTable1(continued)AuthorDateStudyMajorfindingsMondaletal.
2012Analysisofassociationbetweenmalnutrition,socioeconomicstate,immunefactorandamebiasisinthefirstyearoflifeChildrenmalnourishedatbirthweresignificantlymoresusceptibletoE.
histolytica,Cryptosporidium,andETECinfectionsMalnutritionin12monthsispredictedbyprolongeddiarrhealepisodes,intestinalbarrierdysfunction,maternaleducation,andfamilyincomeConcludedthatmalnutritionatbirthpredisposeschildrentoamebiasiswhichaltersintestinalbarrierfunctionandinfluencesnutritionalstatusat12monthsHere,wedescribemajorfindingsfromthelong-termprospectivestudyofapopulationlivinginMirpur,anurbanslumofDhaka,Bangladesh.
Childrenwereobservedeveryotherday.
DiarrhealstoolsweretestedforE.
histolyticausingantigendetectionandPCR.
Bloodwasdrawnevery4monthsfordetectionofserumantibodiesandcytokineresponse,andmonthlystoolsampleswereobtainedtomeasuresecretoryimmunoglobulinAresponse.
Anthropometricswereassessedevery4months774SeminImmunopathol(2012)34:771–785TNF-αactsasapotentchemoattractantforE.
histolytica[40];however,theroleofTNF-αinamebiasisisnotentirelyclear.
Invitro,TNF-αenhanceskillingofE.
histolyticatroph-ozoitesbyneutrophilsandmacrophages[41,42].
Invivo,TNF-αseemstoexacerbatedisease.
BlockingTNF-αpro-ductioninthehumanxenographSCIDmousemodelofamebiasisreducesinflammationandintestinaldamage[43].
Thisfindingwasrecentlyextendedtohumanpopula-tionswhereTNF-αproductionbyamebicantigenstimulat-edPBMCscorrelatedwithincreasedsusceptibilitytoE.
histolyticadiarrhea[44].
IthasbeensuggestedthatIFN-γandprostaglandinE2modulateTNF-αproductionduringinfection[45].
Thus,acomplexarrayoffactorslikelydeter-mineswhetherTNF-αhasaprotectiveordestructiveroleduringamebicinfection.
IFN-γAnalysisofcytokineprofilesintheMirpurstudypopulationhasalsoshownthataboveaverageproductionofIFN-γbutnotIL-5byamebicantigenstimulatedPBMCswasassociatedwithfavorableoutcomesanddecreasedincidenceofE.
histo-lyticainfection.
Whencontrolledfornutritionalstatus,theobservationremainedonlymildlysignificantsuggestingthatIFN-γproductionwasdampenedinmalnourishedsubjects[46].
TheimportanceofIFN-γhasbeenrecapitulatedinmurinevaccinationstudies,whichhavedemonstratedthatIFN-γisasignificantcorrelateofprotectionforE.
histolytica[7,8].
IL-10IL-10-deficientmicearemoresusceptibletoamebicinfec-tion[47],andundernutritionmodifiesIL-10production[48]perhapscontributingtoincreasedsusceptibilityofunder-nourishedindividualstoE.
histolyticadiarrhea.
Overall,IL-10islikelytoplayaroleinconnectingnutritionalstatustoamebiasis.
LeptinTheadipocytokineleptinlinksnutritiontoimmunity.
Im-munefunctionsareimpairedandleptinlevelslowinunder-nourishedindividuals[49].
However,theroleofleptininstarvation-inducedimmunedysregulationhasnotbeenfullyelucidated.
Datafromhumanstudiesintotheeffectsofleptindeficiencyonimmunityinmalnourishedchildrenarecontradictory[50,51].
Nonetheless,miceandhumanswithimpairedleptinsignalingexhibitcompromisedim-munefunctionandaremoresusceptibletomanyinfections,includingamebiasis[52,53]Leptinsignalsthroughthelongformofitsreceptorviathereceptor-associatedJanuskinase2(JAK2).
JAK2phosphorylatesintracellulartyrosineresiduesY985,Y1077,andY1138,whichrecruitSHP2,STAT5,andSTAT3,respec-tively.
SHP2positivelyregulatestheERK/c-fospathway[54].
ActivatedSTAT3upregulatesSOCS3,whichservesasbothafeedbackinhibitorofleptinsignalingandarepressorofapo-ptoticpathways.
Leptinreceptor-mediatedSOCS3signalingalsoregulatesanumberofproinflammatorypathways[55]Leptinsignalingplaysacrucialandwell-characterizedroleinanumberofimmunepathwaysthatareimportantforpro-tectionagainstamebiasis.
LeptinenhancesCCLchemokinesecretioninmurinemacrophages[56],whichpromotesche-motaxisofmonocytes.
Leptinalsoactsdirectlytomobilizemonocytepopulationsvialeptin-dependentPI3K/Aktactiva-tion[57].
Inleptinandleptinreceptor-deficientmice,macro-phagesexhibitphenotypicvariationslikediminishedphagocyticcapacityandloweredcytokineproduction[58,59].
LeptinsignalssecretionofproinflammatorycytokinesTNF-αandIL-6[60]andmayregulatethemonocyticoxidativeburst[61].
Stimulationwithleptinupregulatesproductionofinterleukin1receptorantagonist[62]andIFN-γinducibleprotein[63].
Overall,leptincontributestoaTh1proinflammatoryimmuneresponse,whichiscrucialforclearanceoftrophozoitesduringamebiasis.
Leptinre-ceptornullmice(db/db)exhibitaskewedTh2cytokineprofileanddiminishedTcellproliferation[64],whichcor-relatewithincreasedsusceptibilitytoE.
histolytica.
ProinflammatorychemokinesandcytokinesIL-6,IL1-β,andCXCL1areupregulatedinresponsetoleptin[65],andleptinconcentrationsspikeduringgutinflammation[66].
Theprimaryactionofleptininintestinalepithelialcells(IECs)appearstobemediatedthroughSTAT3,atranscrip-tionfactorknowntoregulateepithelialhomeostasisduringinfectionandinflammation[67,68].
PathogenicstrainsofbacteriainmiceactivateSTAT3incolonicepithelialcells,initiatingaprotectiveTh17mucosalimmuneresponse,characterizedbysecretionofIL-17.
TH-17responsestim-ulatesproductionofIL-6andIL-8,potentmediatorsofinflammationandneutrophilchemotaxis,respectively[69].
Leptinstimulatesanti-apoptoticandproliferativepath-waysthatpromotebarrierfunctionandwoundhealinginepitheliallayers[66,69–73].
Leptinsignalingisanti-apoptoticinavarietyofcelltypes[74].
Dendriticcellsfromdb/dbmicearemorepronetoapoptosispotentiallyasaresultofdecreasedPI3K/Akt,STAT3,andIκB-αsignaling[75,76].
Increasedcaspase-3expressionisobservedincolonicepithelialcellsofdb/dbmice[69].
Thepleiotropicroleofleptininimmunefunctionandenergybalancesupportsthehypothesisthatleptiniscriticaltoanappropriateimmuneresponseinentericdiseaseslikeamebiasisandmayexplainmechanisticallythestrongasso-ciationbetweenundernutritionandamebicdisease.
RecentstudiesinhumansandmicehaverevealedtheimportanceofleptinsignalinginprotectionagainstE.
histolytica.
SeminImmunopathol(2012)34:771–785775Guoetal.
haveshownthatob/obanddb/dbmicearemoresusceptibletoamebicinfectionthanarewild-typecontrols.
Moreover,leptinsignalingintheintestinalepithe-liumalonewassufficienttoconferwild-typeresistance.
Thesiteofleptin-mediatedresistancetoE.
histolyticawasde-terminedusingtissuespecificdeletionsoftheleptinreceptorinE.
histolyticaresistantC57B2/6mice.
Deletionoftheleptinreceptorintheintestinalepitheliumwassufficienttorendermicesusceptibletoamebicinfection.
ExpressionofaleptinreceptordeficientinSTAT5signalingalsorescuedthesusceptiblephenotype.
However,complementationwithreceptorsdeficientinSHP-2orSTAT3didnotrestoreresis-tance,implicatingSTAT3andSHP2butnotSTAT5inleptin-mediatedresistancetoamebiasis[52].
Tofurtherunderstandleptinsignalinginhostdefense,wedevelopedanassaytomeasureamebiccytotoxicityinsinglecells.
Physiologicalconcentrationsofleptinwereprotectiveagainstamebiccytotoxicityincellsthatendog-enouslyorexogenouslyexpressedleptinreceptor.
InHEK293Tcellsexogenouslyexpressingleptinreceptorsig-nalingmutants,onlycellsdeficientintheleptin-dependentSTAT3signalingpathwaylostleptin-mediatedresistancetoamebicchallengewhileSHP2andSTAT5mutantsremainedresistant.
TheQ223Rmutationintheleptinreceptoralsoincreasedamebiccytotoxicityinvitro,andcellsexpressingtheQ223formoftheleptinreceptordisplayedsignificantlyhigherlevelsofactivatedSTAT3inresponsetoleptin[77].
Incombination,theseresultsimplythatleptin-dependentSTAT3activationmediatesdirectcellularresistancetoame-biccytotoxicitywhileleptin-activatedSHP-2signalingmaybeimportantincoordinatingtheglobalimmuneresponsetoamebiasis.
Itisimportanttoemphasizethatthismechanismwaselucidatedinmurineandinvitromodelsofamebicdiseaseandhasyettobeextensivelyvalidatedinhumans.
Innatecell-mediatedimmunitytoE.
histolyticaIntestinalimmunityTheintestinalepitheliumisessentialforprotectionagainstamebiasis.
Approximately90%ofE.
histolyticainfectionsarecommensal,suggestingthatthegutishighlyeffectiveatlimitingprogressiontosymptomaticdisease[78].
Thepro-tectiveroleoftheepitheliuminamebiasisismediatedbythemucousallayer,whichpreventsamebafromadheringtoIECs.
IECsandtightgapjunctionsprovideasecondphys-icalbarriertoinvasion.
Asthesebarriersbegintofail,epithelialcellsrecruitandactivateothereffectorsofinnateimmunitythroughchemokineandcytokinerelease.
Resi-dentmicrobesmayalsoaffectE.
histolyticavirulenceandintestinalinflammation.
AsimplifiedmodelofintestinalinvasionbyE.
histolyticatrophozoitesisdepictedinFig.
1.
MucousallayerMucusoffersthefirstlayerofprotectionagainstinvadingtrophozoitesintheintestine.
Gobletcellssecretemucins,whichsolubilizetoformagelatinousbarrierbetweenthelumenandepitheliumformingabarrieragainstentericpathogens.
StudiesbyChadeeetal.
haveshownthatsecret-edcolonicmucinsarecriticalforgastricmucosalrepair[79]andresistancetobacterialcolitis[80].
TheprocessbywhichinvasiveE.
histolyticatrophozoitespenetratetheprotectivemucousbarriertocontactandkillsub-mucousalepithelialcellsiswellunderstood.
TheGal/GalNacadherencelectinofE.
histolyticaadherestoglycosylatedcomponentsofthemucousalmatrix[81].
Next,parasiticcysteineproteasescleavemucinsatsitesoflowglycosylation.
Degradingmucinsandothercomponentsofthemucouslayerallowsamebatobindsub-mucousalcells,inducecelldeath,andpenetratetheepithelium[82].
RecentworkinMUC2knockoutmicehashighlightedthespecificimportanceofmucinsasprotectivemoleculesagainstE.
histolytica.
Bergstrometal.
demonstratedthatMUC2deficientmicechallengedwithE.
histolyticadis-playedanacuteproinflammatoryresponse,characterizedbyincreasedgrosspathology,luminalTNF-αandIFN-γ,andalteredexpressionofgapjunctionproteins[80].
ResultsfromChadeeetal.
recentlyrecapitulatedthisfindingincolonicloopsfromMUC2-deficientmiceandhavegoneontoshowthattrophozoitesalsorequireMUC2toinvadetheepithelium.
E.
histolyticaappearstoinducehigherMUC2expressionandtrophozoitesbecometrappedinthemucouscoatingofthecolonicepitheliumpresumablybe-foreinvading.
E.
histolyticacysteineprotease5(CP5)directlycleavesMUC2.
Expressionofthisproteaselikelyallowstheparasitetoovercometheprotectivemucousbarrier[83].
WhenCP5issilenced,trophozoitesfailtopenetratethecoloniclaminapropriaanddonotinduceproinflammatorycytokinesecre-tioninexvivomodelsofamebiccolonicinvasion[84].
TheseresultssuggestthatdisassociationofE.
histolyticafromthecolonicepitheliumbymucous,preventsadamag-inginflammatoryresponse.
MicrobiomeThemicrobesthatcolonizetheshallowsectionofthemu-cosallayeroftheintestinalepitheliummayimprovebarrierfunctionbyinhibitingadherenceofpathogenicspeciesandcompetingfornutrients.
Entamoebaarenaturallycolonizedbybacteria,whichaffectparasitevirulence[85].
Theaxeni-zationofEntamoebaaltersthesurfaceantigensofthepar-asite[86].
Astrophozoitesfeedprimarilyonresidentgutflora,itisplausiblethatdifferencesinhostmicrobialecol-ogycontributetotheinitiationofavirulentphenotype.
776SeminImmunopathol(2012)34:771–785Already,reductioninlactobacillusspecieshasbeenob-servedinALApatients[87].
Numerousstudieshaveshownthatmicrobialcomposi-tionislinkedtonutritionalstatusanditscompositioncanalternutrientabsorptionandbioavailability[88–91].
Nutri-entdeprivationaltershostsusceptibilitybutmayalsocon-tributetovirulenceoftheparasite.
Severalmicro-andmacronutrientshavebeenshowntoinfluenceE.
histolyticavirulenceincludingcalciumviaregulationofURE3BP[19],glucose[92],cholesterol[93],andiron[94].
Theemergingfieldofmicrobialmetagenomicsislikelytocontinuetorevealanimportantroleforthegutmicro-biomeinthepathogenesisofE.
histolyticaviamodulationoftheimmuneresponseandvirulenceoftheparasiteitself.
AntimicrobialcompoundsSecretedimmunoglobulins,defensins,andcathalecidinsre-inforcemucousalbarrierfunction,activelypreventingpath-ogeniccolonizationbyameba.
Cathelicidins,antimicrobialpeptidesresidentinthegutmucosa,areimportantmediatorsofmammalianinnateimmunityandwhileE.
histolyticatrophozoitesactivatecathelicidinproductioninbothhumanandmouseintestinalepitheliallayers,theyareresistanttotheircytolyticeffect.
AmebiccysteineproteasescleavethecathelicidinsLL-37andCRAMPbutthefragmentsretainbactericidalactivity[95].
Fourregeneratinggene(REG)C-typelectinshavebeencharacterizedinhumans.
Overall,REGsfunctiontoinducecellularproliferationandinhibitapoptosis.
REGIαseemstohaveaphysiologicalroleinthegastricmucosaandREGsarefoundthroughoutthegastrointestinalsystem.
REGIα,REGIβ,andREGIIImRNAsareoverexpressedinthecolonininflammatoryboweldisease,Crohn'sdisease,andulcerativecolitis[96].
Microarrayanalysisofintestinalbi-opsiesfrompatientsinearlyandrecoverystagesofamebi-asishasshownthatREGIαandREGIβarehighlyupregulatedduringacuteinfectionrelativetoconvalescenceandimmunohistochemicalstudiesconfirmthisresult[97].
Thus,REGslikelyhaveafunctionalroleinregenerationoftheintestinalepitheliumafteramebicdisease.
TherecentlydiscoveredantimicrobialfunctionofREGIII[98]suggeststhatREGproteinsmayhavedualfunctionsinintestinalbarrierreinforcementandamebickilling[99].
IntestinalepithelialcellsIECsrecognizemultipleamebicantigensanddirecttheimmuneresponseintheacutephaseofamebiasis.
Itisgenerallythoughtthattheepitheliumdownregulatespatternrecognitionreceptors(PRR)topreventchronicinflamma-tioninresponsetoresidentmicrobialantigens[9].
Interest-ingly,stimulationofIEClayerswiththeadherencelectinofE.
histolyticaincreasesexpressionofTLR2,whichrecog-nizesamebicLPPGandactivatesNFκB-regulatedsecretionofproinflammatorycytokinesfromhumanmonocytes,mac-rophages,anddendriticcells[100].
Invitro,whenstimulat-edwithamebicantigens,IECssecreteTNF-α,IL-6,GROa,andGM-CSF[101].
E.
histolyticaDNAhasbeenshowntoactivateTLR9[100]whichrecognizesunmethylatedCpGdinucleotides[101].
Additionally,CP5bindstoα(V)β(3)integrinonCaco-2coloniccellsviatheRGBmotifandstimulatesanNFκB-mediatedproinflammatoryresponsesviaPI3K/Akt[102,103].
IntheSCIDmouse–humanxeno-graftmodelofALA,amebicchallengeincreasedIL-8andIL-1βsecretionpresumablypromotingacuteneutrophilicinfiltration[104].
IECdeathitselfmayrecruitandstimulateimmunecells.
Ithaslongbeenthoughtthatcontact-Fig.
1Intestinalimmunity.
E.
histolyticatrophozoitesencounteralayeredhostdefenseastheyinvadetheintestine.
Intestinalepithelialcells(IECs)directaninflammatoryresponsetoinvasiveamebabyrecruitingneutrophils,macrophages,andotherimmunecells.
Trophozoitesthatsurvivemayprogresstothecirculatorysystemwheretheyencounterhumoralantibodiesandmustevadecomplement-mediatedlysisSeminImmunopathol(2012)34:771–785777dependentkillingbytrophozoiteswascanonicallyapoptotic.
Amebiccytotoxicityinducesmembraneblebbing,TUNELpositivity,surfaceexposureofphosphatidylserine,andcas-paseactivation.
Furthermore,caspaseinhibitionandoverex-pressionofanti-apoptoticeffectorslikeBcl-2areprotectiveinmousemodelsofdisease[105].
Pyropototicandnecroticdeathareincreasinglyrecognizedasimportantforamebiccytotoxicityashostcellsexperiencearapidlossofmembraneintegritynotconsistentwithcanonicalapoptosis.
Theselatterdeathpathwaysmaycontributemorepotentlytoinflammationintheintestinethroughreleaseofhostcellularcomponents.
AmebicinvasionthroughtheIEClayerandintothelaminapropriamayoccurpassivelythroughsmallgapsresultingfrominflammation[101].
Activepenetrationislikelymediatedbyamebicproteasesthatcleavegapjunc-tions,facilitatingtransepithelialmovement[14].
Interesting-ly,E.
histolyticahasadaptedtodampentheNFκB-mediatedinflammatoryresponseofIECsviainductionofheatshockprotein27(hsp27),whichsuppressesNFκBtranscriptionalregulation[106].
TheseobservationssuggestthattheintestinalepitheliumiscrucialbarriertoinfectionbyE.
histolytica.
PathogenicamebamustbreachthemucousalmatrixtoaccessIECs,whosePRRsrecognizeandrespondtoamebicinvasion.
Attheonsetofacuteamebiccolitis,ulcersformandinflam-matorysignalsfromIECsrecruiteffectorsofinnateimmu-nitytothesiteofdangerwhileinitiatinganadaptiveresponse.
Thedegreeofimmuneactivationintheintestinalepitheliumlikelydeterminestheprogressionandseverityofamebiasisasmuchoftheresultanttissuedamageismediat-edbythehostinflammatoryresponse.
Post-epithelialimmunityNeutrophilsTheprimaryroleofneutrophilsininnateim-munityistodestroyandengulfpathogensinaffectedtis-sues.
NeutrophilextracellularNADPHoxidasegeneratesreactiveoxygenspeciesthataretoxictoinvadingpathogens.
Neutrophilsalsoexpresssurfacereceptorsforopsoninsthatfacilitatephagocytosisofpathogensmarkedbyotherbranchesoftheimmunesystemfordestruction[115].
Neu-trophilsalsoexudeextracellulartraps(NETs)composedofchromatinandserineproteasesthatensnareandneutralizepathogens[116].
AroleforNETsinthepathogenesisofamebiasishasyettobecharacterized.
Trophozoitesthatbreachtheepithelialbarrierelicitarapidinfiltrationofneutrophilstothesiteofinfectioninearlystagesofintestinaldisease[107,108].
IECssecreteIL-8,apotentneutrophilchemoattractant,whenexposedtoE.
histolytica[109].
ActivationandtranslocationmayalsooccurinresponsetoanaphylotoxinsC5aandC3aofthecomplementsystemthoughamebicproteasescleaveandinactivatebotheffectors[110].
Itisgenerallyacceptedthatneutrophilsareresponsibleformuchofthetissuedamageassociatedwithamebiccolitis.
Whileneutrophilsdopossessamebacidalactivity,whichlike-lyaidsinpathogenclearanceforasymptomaticinfections,virulenttrophozoiteshaveadaptedeffectivemechanismsofresistanceandcounterattack.
NeutrophilsincultureamplifythecytopathogenicityofE.
histoltyicatrophozoitesonepithe-lialcells[111]likelyduetoneutrophildegranulationoftoxiccellularcomponents[112].
Leptindeficiencyandgeneticdifferencesinleptinsig-nalingpathwaysmayalsoaffectneutrophilresponsetoamebicinvasion.
Neutrophilsexpressashortformoftheleptinreceptorthatissufficientforleptinsignaling,whichinhibitsapoptoticpathwaysandactsasachemoattractant[54,113],andmaymodulateoxidativecapacityinneutro-philsduringinfection[114].
Thereissomeevidencefrommouseandinvitromodelsofamebiasisthatneutrophilsplayaprotectiveroleearlyininvasion[105,117,118].
NeutrophildepletionintheSCIDmousemodelexacerbatesintestinaldiseaseandliverab-scessformation[105,117,118].
However,theGR-1anti-bodiesusedinthesestudiesmayalsodepletemonocytesandeosinophils.
Inearlystagesofhepaticamebiasis,neutrophilsarethemajoreffectorsoftheacuteinflammatoryresponse,[119]butcanbebothprotective[120]anddestructive[111].
NeutrophilsprimedwithIFN-γ,TNF-α,orLPSdoexhibitamebicidalactivity[112].
However,E.
histolyticahasproventobefarmoreeffectiveatkillingneutrophils,withmorevirulentstrainskillingataratioof1trophozoiteto3,000neutrophils[112].
E.
histolyticatrophozoitesinduceneutrophilapoptosisdirectlybyengagingwithintegrinsandbyactivatingNADPHoxidase[121,122].
Invasiveamebicstrainsareresistanttotherespiratoryburstasanamebicperoxidoredoxinprotectstroph-ozoitesfromreactiveoxygenspecies[121,122].
Neutrophilcorpsesarequicklyphagocytosedbytrophozoites[112].
Figure2summarizessomeofwhatisknownabouttheroleofneutrophilsinhostdefenseagainstE.
histolytica.
MacrophagesMacrophagesplayimportantrolesinthepathogenesisofbothintestinalandhepaticamebiasis.
Macro-phagesareactivatedbyE.
histolyticatrophozoitesandareamebicidalwhenstimulatedwithCSF,IFN-γ,andTNF-α[112].
TheamebicsurfacecomponentLPPGactivatesaclas-sicalinflammatorymacrophageresponseuponTLR2/6andTLR4binding[123].
E.
histolyticaDNAalsopromotesmacrophageactivationbyTLR9binding[104].
Furthermore,macrophagesupregulateTLR2inresponsetotheamebicgalactose-specificlectin[104].
Macrophageamebicidalactiv-ityismediatedbynitricoxidesynthase(NOS)[124].
NOinhibitsimportantamebicvirulencefactorsincludingthecys-teineproteasesandalcoholdehydrogenase2[125].
Invasiveamebaareabletoregulatemacrophageresponsesinanumberofways.
Analogoustotheirinteractionwithneutrophils,trophozoitesinhibittherespiratoryburstof778SeminImmunopathol(2012)34:771–785macrophages.
Withmacrophages,thisfunctiondependsonanamebicarginasethatcompetitivelyconvertsL-arginine,asub-strateofmacrophageNOS,toL-ornithine[126].
Amebaalsomodulatemacrophagecytokinesecretion[127,128].
Produc-tionofTNF-αisblockedbyamebicdegradationofc-fosandTNF-αtranscripts[129].
E.
histolyticaproducesacyclooxy-genase2,theprostaglandinproductofwhichhashost-immunomodulatoryfunction[130,131].
Prostaglandintrig-gersacAMPspikeinmacrophages.
ThissignalactivatesPKAandIL-8secretion[131].
PKAinhibitsexpressionofTh1cytokinesandPKC-mediatedNOsynthesis[130].
Finally,themonocytelocomotioninhibitoryfactor(MLIF)ofE.
his-tolyticahasbeenimplicatedinalteringmacrophagefunction.
MLIFinhibitsNOandproinflammatorycytokineproductionwhilestimulatingthesecretionofIL-10,animportantanti-inflammatoryeffector[132,133].
Macrophagesrepresentasecondarylineofdefenseafterthemassiveinfiltrationofneutrophilsduringacutehepaticamebiasis.
NOSisprotectiveanditsliverspecificdeletionincreasesseverityofALAininfectedmice[124].
IFN-γisalsoimportantformacrophage-mediatedprotectionagainsttrophozoites.
BlockingIFN-γreceptorsincreasessusceptibil-itytoALAinmice[134].
Classicallyactivatedmacrophagesareeffectiveatclearingtrophozoitesandevenalternativeactivationcanpromotewoundrepairandrecruitmentofadap-tiveimmunecells[135].
E.
histolyticasuppressesbothacti-vatedclassesofmacrophages,underminingcell-mediatedimmunityandleadingtoestablishmentofchronicALA[127].
Figure3summarizessomeofwhatisknownabouttheroleofmacrophagesinhostdefenseagainstE.
histolytica.
NaturalkillerandnaturalkillerTcellsNaturalkillercellsdonotrequireprimingtobefunctionallycytotoxicagainstpathogens.
Theircanonicallymyopic"killer"functionhasbeenchallengedinrecentyearsasmorestudieselucidatediverserolesforNKcellsinimmuneregulation[136].
ActivatedNKcellssecreteIFN-γandTNF-α,importantfactorsinhost-immunitytoamebiasis.
NKcellsinfiltratetheliverduringtheacutephaseofexperimentalALAinmousemodels[137].
VirulenttrophozoitesstimulateNKcellcyto-toxicitymoreeffectivelythandonon-pathogenicstrains[138].
E.
histolyticamayregulateNKcellsthroughCP-mediatedcleavageofC5a,ananaphylotoxinknowntoacti-vateNKcellsduringsepsis[139].
NKTcellsexpressmarkersthatdelineateNKcellsbutco-expressαβTCR.
TheydifferfrommostT-cellpopulationsinthattheyrecognizeantigenspresentedbyCD1dinsteadofMHCassociatedantigens.
InvariantNKT(iNKT)cellsareasubsetofNKTcellsthatexpressinvariantα-TCRandproducelargeamountsofpro-andanti-inflammatorycyto-kinesuponactivation.
iNKTcellsareactivatedtoproduceIFN-γbutnotIL-4inaTLR/CD1d-dependentmanner[140].
StudiesinmousemodelsofALAsuggestthatclear-anceoftrophozoitesduringearlystagesofhepaticcoloni-zationisdependentonNKTcellactivity.
MicelackingNKTcellsarehighlysusceptibletoALA[141].
ClearancewasassociatedwithelevatedIFN-γproduction.
Likeneutrophils,NKTcellslikelyhaveacontext-dependenteffectondiseaseprogression.
TheirresponsetoamebicantigensmaycontributetopolarizingtheimmuneresponsetowardtheTh1phenotypethoughttobeprotectiveinamebiasis.
IntermsofALA,aftertheacutephaseofhepaticinfiltration,particularlyresistanttrophozoitessur-viveandexpand.
Atthisstage,iNKTcellsmayincreasetissuedamagebypropagatingdeleteriousinflammatorysig-nalsandstimulatingneutrophilchemotaxis.
Fig.
2NeutrophilsrecruitedtothesiteofE.
histolyticainfectiontargetpathogensbysecretingNADPHoxidase,anenzymethatcatalyzestheproductionofreactiveoxygenspecies.
Interleukins,interferons,andtumornecrosisfactoralphaprimeandrecruitmoreimmunecells.
Theparasiterespondswithperoxiredoxinandcysteineproteases,whichcleaveandneutralizehostfactorstomakewayforlectin-mediatedadherenceandeffectorsofamebiccytotoxicitySeminImmunopathol(2012)34:771–785779ComplementThepathogenesisofextraintestinalamebiasisdependsonevasionofhumoralinnateimmunitybyE.
histolyticaandbothalternativeandclassicalbranchesofthecomplementsystemareinvolved.
Allcomplementpath-waysconvergetoproduceapathogen-associatedC3-convertasethatcleavesC3intotheopsoninC3bandtheinflammatoryanaphylotoxinC3a.
Theproteolyticcascadeculminatesinpathogenlysisviadepositionandpolymeri-zationofpore-formingmembraneattackcomplexes(MACs)onopsonizedinvaders[142].
E.
histolyticacanactivateboththealternativebranchandtheserum-antibody-dependentclassicalbranchofthecom-plementcascade[143,144].
AneutralE.
histolyticacysteineproteasecleavesC3toanactiveisoformofC3b[144].
Introphozoitesthataresusceptibletocomplement,C3b,acleavageproductofamebicCP,successfullyrecruitstermi-naleffectorsofthecascaderesultinginMACformationandparasitelysis[145].
Trophozoitesisolatedfromasymptom-aticcasesarereadilylysedinacomplement-dependentmanner,butpathogenictrophozoitesfrompatientswithamebicliverabscessorinvasivecolitisareresistanttocomplement-mediatedkilling[146].
ThesestudiessuggestthatcomplementisanimportantbarrieragainstinvasionbyE.
histolytica,presumablycontributingtothelowincidenceofextraintestinaldisease.
Furthermore,amebicresistancetocomplement-mediatedkillingrepresentsanadaptationuniquetotrophozoitesthatcauseextraintestinaldisease.
Hostcellspreventtheirowncomplement-mediatedlysisbyexpressingsurfaceCD59.
ExpressionofCD59inhibitsopso-nizationandMACformationonhostcells[147].
TheGal/GalNaclectinofE.
histolyticaisamultisubunitGPI-anchoredcomplexessentialforparasiterecognitionofhostsurfaces.
Bragaetal.
showedthatthislectincontainsaCD59-likeregionthatinhibitsMACformationandprotectstheparasitefromcomplement-mediatedlysis[148].
Inagreementwiththeseresults,globalinhibitionofGPIanchorformationleavespreviouslyresistantE.
histolyticatrophozoitessusceptibletocomplement-mediatedlysis[149].
OtherCD59-likeamebicproteinsmayalsocontributetoresistance.
Venturaetal.
recentlyidentifiedanovel21kDaamebicsurfaceproteinthatreactswithhumananti-CD59[150].
However,itsfunctional-ityasaninhibitorofMACformationanditsmoleculariden-tityhaveyettobeelucidated.
Amebiccysteineproteasesalsoplayaroleincomplementevasion.
ThesurfaceEhCPimplicatedinAPactivationbyC3cleavage[144]alsocleavesandinactivatestheinflammatoryanaphylotoxinsC3aandC5a[110].
ThusCPsappearstoplayopposingrolesinevasionofhostimmunitybyE.
histolytica,ontheonehandactivatingcomplementandontheothersuppressingnumerousinflammatorypathwaysdownstreamFig.
3MacrophagesrecruitedtothesiteofE.
histolyticainfectionareclassicallyactivatedbyinterferongammaandothercytokines.
TheyproduceNOtokillpathogensandsecreteIL-10andIL-8,promotinggutbarrierfunctionandneutrophilrecruitment,respectively.
Thepar-asiticarginasecompetitivelyinhibitsandtheMLIFaltersexpressionofhostnitrousoxidesynthase.
E.
histolyticaCpGDNAactivatesTLR9780SeminImmunopathol(2012)34:771–785ofC3aandC5a.
Athirdmechanismofcomplementresistancemayinvolveenrichmentoflypophosphoglycan(LPG)intheouterleafletoftheE.
hystolyticaplasmamembrane.
WhileLPGisproducedinthepathogenicE.
histolytica,significantlevelsarenotobservedinthenon-pathogenicstrainE.
dispar[151].
LPGprotectsLeishmaniafromcomplement-mediatedlysisbyimpedingthedepositionofproteolyticcomplexesandpreventingMACformation[152].
E.
histolyticatrophozoitesmayemployasimilarstrategy.
ALAisapproximatelyfivetimesmoreprevalentinmenthaninwomen,suggestingasexualdimorphisminsusceptibil-itytoextraintestinalamebiasis[153].
Differencesinthecyto-pathogeniceffectsofcomplementonE.
histolyticabetweenthetwosexeshavebeenimplicated[9].
Nonimmunefemaleserumkillstrophozoitesmoreeffectivelythandoesnonimmunemaleserum[154].
However,itisunlikelythatcomplementissolelyresponsibleforthedifferenceinsusceptibilitytoextraintestinaldisease.
Interestingly,thesexualdimorphismobservedinhumanswascorroboratedinmousestudies[134].
FemaleB6miceweremoreeffectivethanmalesatclearinginvasivetroph-ozoitesfromtheliverinaSCIDmousemodelofALA[141].
ClearancewasassociatedwitharapidNKTcellresponseandhighIFN-γproduction[141].
Malemiceclearedinfectionslesseffectively,IFN-γproductionwaslessrobust,andsignificantIL-4productionwasobserved.
ConclusionsGenerationsofresearchhaveprovidedatremendousbodyofknowledgeabouthostimmunitytoamebiasis.
Recentworkhashighlightedtheimportanceoftheparasitegenomeandtran-scriptomeinprogressiontoavirulentphenotype.
Significantprogresshasalsobeenmadeinvaccineanddrugdevelopmentandwenowhaveabetterunderstandingofenvironmentalfactorsthatcontributetoamebiasis.
ThecomplexityoftheinteractionbetweenE.
histolyticatrophozoitesandhost-innateimmunitycontinuestomakeidentificationoffactorsthatinflu-encesusceptibilitytoinfectionafascinatingchallenge.
Effec-tiveapproacheshaveunitedpowerfultechniquesinpopulationgenetics,immunology,andepidemiologytoprobethefullcomplexityoftherelationship.
Usingworkinhumanpopula-tionstoinformmouseandinvitrostudiesofamebiasishasbeenparticularlyeffective,allowingustofocusonhostandparasitefactorsproventoeffectimmunityinhumans.
References1.
HaqueR,MondalD,KirkpatrickBDetal(2003)EpidemiologicandclinicalcharacteristicsofacutediarrheawithemphasisonEntamoebahistolyticainfectionsinpreschoolchildreninanurbanslumofDhaka,Bangladesh.
AmJTropMedHyg69(4):398–4052.
Espinosa-CantellanoM,Martínez-PalomoA(2000)Pathogenesisofintestinalamebiasis:frommoleculestodisease.
ClinMicrobiolRev13(2):318–3313.
BlackRE,AllenLH,BhuttaZAetal(2008)Maternalandchildundernutrition:globalandregionalexposuresandhealthconse-quences.
Lancet371(9608):243–2604.
PetriWAJr,ChaudhryO,HaqueR,HouptE(2006)Adherence-blockingvaccineforamebiasis.
ArchivesofMedicalResearch37(2):287–2905.
HaqueR,AliIM,SackRB(2001)AmebiasisandmucosalIgAantibodyagainsttheEntamoebahistolyticaadherencelectininBangladeshichildren.
JInfectDis183(12):1787–17936.
AbdAllaMD,WolfR,WhiteGL(2012)Efficacyofagal-lectinsubunitvaccineagainstexperimentalEntamoebahistolyticainfec-tionandcolitisinbaboons(Papiosp.
).
Vaccine30(20):3068–30757.
GuoX,BarrosoL,BeckerSMetal(2009)ProtectionagainstintestinalamebiasisbyarecombinantvaccineistransferablebyTcellsandmediatedbygammainterferon.
InfectImmun77(9):3909–39188.
GuoX,BarrosoL,LyerlyDM,PetriWA,HouptER(2011)CD4+andCD8+Tcell-andIL-17-mediatedprotectionagainstEnt-amoebahistolyticainducedbyarecombinantvaccine.
Vaccine29(4):772–7779.
MortimerL,ChadeeK(2010)TheimmunopathogenesisofEnt-amoebahistolytica.
ExpParasitol126(3):366–38010.
GuoX,HouptE,PetriWAJr(2007)Crosstalkattheinitialencounter:interplaybetweenhostdefenseandamebasurvivalstrategies.
CurrOpinImmunol19(4):376–38411.
BillerL,DavisPH,TillackMetal(2010)DifferencesinthetranscriptomesignaturesoftwogeneticallyrelatedEntamoebahistolyticacelllinesderivedfromthesameisolatewithdifferentpathogenicproperties.
BMCGenomics11:6312.
MirelmanD,AnbarM,BrachaR(2008)TrophozoitesofEnt-amoebahistolyticaepigeneticallysilencedinseveralgenesarevirulence-attenuated.
Parasite15(3):266–27413.
BrachaR,NuchamowitzY,AnbarM,MirelmanD(2006)Tran-scriptionalsilencingofmultiplegenesintrophozoitesofEnt-amoebahistolytica.
PLoSPathog2(5):e4814.
QueX,ReedSL(2000)Cysteineproteinasesandthepathogen-esisofamebiasis.
ClinMicrobiolRev13(2):196–20615.
CostaCA,NunesC,FerreiraAJ,GomesMA,CaliariMV(2010)EntamoebahistolyticaandE.
dispartrophozoitesintheliverofhamsters:invivobindingofantibodiesandcomplement.
ParasitVectors3:2316.
AliIKM,ClarkCG,PetriWA(2008)Molecularepidemiologyofamebiasis.
InfectGenetEvol8(5):698–70717.
AliIKM,Solaymani-MohammadiS,AkhterJetal(2008)TissueinvasionbyEntamoebahistolytica:evidenceofgeneticselectionand/orDNAreorganizationeventsinorgantropism.
PLoSNeglTropDis2(4):e21918.
GilchristCA,HouptE,TrapaidzeNetal(2006)ImpactofintestinalcolonizationandinvasionontheEntamoebahistolyticatranscriptome.
MolBiochemParasitol147(2):163–17619.
MorenoH,LinfordAS,GilchristCA,PetriWA(2010)Phospholipid-bindingproteinEhC2Amediatescalcium-dependenttranslocationoftranscriptionfactorURE3-BPtotheplasmamem-braneofEntamoebahistolytica.
EukaryotCell9(5):695–70420.
GilchristCA,MooreES,ZhangYetal(2010)RegulationofvirulenceofEntamoebahistolyticabytheURE3-BPtranscriptionfactor.
mBio1(1):e00057-1021.
TengkuSA,NorhayatiM(2011)Publichealthandclinicalim-portanceofamoebiasisinMalaysia:areview.
TropBiomed28(2):194–22222.
SinghA,HouptE,PetriWA(2009)Rapiddiagnosisofintestinalparasiticprotozoa,withafocusonEntamoebahistolytica.
Inter-discipPerspectInfectDis2009:547090SeminImmunopathol(2012)34:771–78578123.
BlackRE,BrownKH,BeckerS(1984)Malnutritionisadeter-miningfactorindiarrhealduration,butnotincidence,amongyoungchildreninalongitudinalstudyinruralBangladesh.
AmJClinNutr39(1):87–9424.
YoonPW,BlackRE,MoultonLH,BeckerS(1997)Theeffectofmalnutritionontheriskofdiarrhealandrespiratorymortalityinchildren<2yofageinCebu,Philippines.
AmJClinNutr65(4):1070–107725.
MondalD,HaqueR,SackRB,KirkpatrickBD,PetriWA(2009)Attributionofmalnutritiontocause-specificdiarrhealillness:evidencefromaprospectivestudyofpreschoolchildreninMir-pur,Dhaka,Bangladesh.
AmJTropMedHyg80(5):824–82626.
MondalD,MinakJ,AlamMetal(2012)Contributionofentericinfection,alteredintestinalbarrierfunction,andmaternalmalnu-tritiontoinfantmalnutritioninBangladesh.
ClinInfectDis54(2):185–19227.
ChandraRK(1992)Protein-energymalnutritionandimmunolog-icalresponses.
JNutr122(3):597–60028.
ChristadossP,TalalN,LindstromJ,FernandesG(1984)Sup-pressionofcellularandhumoralimmunitytoT-dependentanti-gensbycalorierestriction.
CellImmunol88(1):1–829.
GuerrantRL,SchorlingJB,McAuliffeJF,DeSouzaMA(1992)Diarrheaasacauseandaneffectofmalnutrition:diarrheapre-ventscatch-upgrowthandmalnutritionincreasesdiarrheafre-quencyandduration.
AmJTropMedHyg47(1Suppl):28–3530.
GuerrantRL,OriáRB,MooreSR,OriáMO,LimaAA(2008)Malnutritionasanentericinfectiousdiseasewithlong-termeffectsonchilddevelopment.
NutrRev66(9):487–50531.
SavinoW,DardenneM(2010)Nutritionalimbalancesandinfec-tionsaffectthethymus:consequencesonT-cell-mediatedim-muneresponses.
ProcNutrSoc69(04):636–64332.
AabyP,MarxC,TrautnerSetal(2002)Thymussizeatbirthisassociatedwithinfantmortality:acommunitystudyfromGuinea-Bissau.
ActaPaediatrica91(6):698–70333.
SchaibleUE,KaufmannSHE(2007)Malnutritionandinfection:complexmechanismsandglobalimpacts.
PLoSMed4(5):e11534.
MondalD,PetriWAJr,SackRB,KirkpatrickBD,HaqueR(2006)Entamoebahistolytica-associateddiarrhealillnessisneg-ativelyassociatedwiththegrowthofpreschoolchildren:evi-dencefromaprospectivestudy.
TransactionsoftheRoyalSocietyofTropicalMedicineandHygiene100(11):1032–103835.
HaqueR,MondalD,KarimAetal(2009)Prospectivecase–controlstudyoftheassociationbetweencommonentericprotozoalpara-sitesanddiarrheainBangladesh.
ClinInfectDis48(9):1191–119736.
DuggalP,HaqueR,RoySetal(2004)InfluenceofhumanleukocyteantigenclassIIallelesonsusceptibilitytoEntamoebahistolyticainfectioninBangladeshichildren.
JInfectDis189(3):520–52637.
DuggalP,GuoX,HaqueRetal(2011)AmutationintheleptinreceptorisassociatedwithEntamoebahistolyticainfectioninchildren.
JClinInvest121(3):1191–119838.
LocksleyRM,KilleenN,LenardoMJ(2001)TheTNFandTNFreceptorsuperfamilies:integratingmammalianbiology.
Cell104(4):487–50139.
SéguinR,MannBJ,KellerK,ChadeeK(1995)Identificationofthegalactose-adherencelectinepitopesofEntamoebahistolyticathatstimulatetumornecrosisfactor-alphaproductionbymacro-phages.
ProcNatlAcadSciUSA92(26):12175–1217940.
BlazquezS,ZimmerC,GuigonGetal(2006)HumantumornecrosisfactorisachemoattractantfortheparasiteEntamoebahistolytica.
InfectImmun74(2):1407–141141.
DenisM,ChadeeK(1989)Cytokineactivationofmurinemacro-phagesforinvitrokillingofEntamoebahistolyticatrophozoites.
InfectImmun57(6):1750–175642.
SéguinR,MannBJ,KellerK,ChadeeK(1997)Thetumornecrosisfactoralpha-stimulatingregionofgalactose-inhibitablelectinofEntamoebahistolyticaactivatesgammainterferon-primedmacrophagesforamebicidalactivitymediatedbynitricoxide.
InfectImmun65(7):2522–252743.
ZhangZ,MahajanS,ZhangX,StanleySL(2003)TumornecrosisfactoralphaisakeymediatorofgutinflammationseeninamebiccolitisinhumanintestineintheSCIDmouse–humanintestinalxenograftmodelofdisease.
InfectImmun71(9):5355–535944.
PetersonKM,ShuJ,DuggalPetal(2010)AssociationbetweenTNF-αandEntamoebahistolyticadiarrhea.
AmJTropMedHyg82(4):620–62545.
WangW,KellerK,ChadeeK(1992)ModulationoftumornecrosisfactorproductionbymacrophagesinEntamoebahisto-lyticainfection.
InfectImmun60(8):3169–317446.
HaqueR,MondalD,ShuJetal(2007)Correlationofinterferon-Γproductionbyperipheralbloodmononuclearcellswithchild-hoodmalnutritionandsusceptibilitytoamebiasis.
AmJTropMedHyg76(2):340–34447.
HamanoS,AsgharpourA,StroupSEetal(2006)ResistanceofC57BL/6micetoamoebiasisismediatedbynonhemopoieticcellsbutrequireshemopoieticIL-10production.
JImmunol177(2):1208–121348.
VinoloMAR,FockRA,CrismaARetal(2008)Protein-energymalnutritionmodifiestheproductionofinterleukin-10inre-sponsetolipopolysaccharide(LPS)inamurinemodel.
JNutrSciVitaminol54(5):371–37749.
FaggioniR,FeingoldKR,GrunfeldC(2001)Leptinregulationoftheimmuneresponseandtheimmunodeficiencyofmalnutrition.
FASEBJ15(14):2565–257150.
PalacioA,LopezM,Perez-BravoF,MonkebergF,SchlesingerL(2002)Leptinlevelsareassociatedwithimmuneresponseinmalnourishedinfants.
JCEM87(7):3040–304651.
MooreSE,MorganG,CollinsonACetal(2002)Leptin,malnu-trition,andimmuneresponseinruralGambianchildren.
ArchDisChild87(3):192–19752.
GuoX,RobertsMR,BeckerSMetal(2011)LeptinsignalinginintestinalepitheliummediatesresistancetoentericinfectionbyEntamoebahistolytica.
MucosalImmunol4(3):294–30353.
MooreSI,HuffnagleGB,ChenG-H,WhiteES,MancusoP(2003)LeptinmodulatesneutrophilphagocytosisofKlebsiellapneumoniae.
InfectImmun71(7):4182–418554.
BrunoA,ConusS,SchmidI,SimonH-U(2005)Apoptoticpathwaysareinhibitedbyleptinreceptoractivationinneutro-phils.
JImmunol174(12):8090–809655.
BjrbkC,El-HaschimiK,FrantzJD,FlierJS(1999)TheroleofSOCS-3inleptinsignalingandleptinresistance.
JBiolChem274(42):30059–3006556.
KiguchiN,MaedaT,KobayashiY,FukazawaY,KishiokaS(2009)LeptinenhancesCC-chemokineligandexpressionincul-turedmurinemacrophage.
BiochemBiophysResCommun384(3):311–31557.
GruenML,HaoM,PistonDW,HastyAH(2007)Leptinrequirescanonicalmigratorysignalingpathwaysforinductionofmono-cyteandmacrophagechemotaxis.
AmJPhysiolCellPhysiol293(5):C1481–C148858.
LeeF-YJ,LiY,YangEKetal(1999)Phenotypicabnormalitiesinmacrophagesfromleptin-deficient,obesemice.
AmJPhysiolCellPhysiol276(2):C386–C39459.
LoffredaS,YangSQ,LinHZetal(1998)Leptinregulatesproinflammatoryimmuneresponses.
FASEBJ12(1):57–6560.
Santos-AlvarezJ,GobernaR,Sánchez-MargaletV(1999)Hu-manleptinstimulatesproliferationandactivationofhumancir-culatingmonocytes.
CellImmunol194(1):6–1161.
Sánchez-PozoC,Rodriguez-BaoJ,Domínguez-CastellanoAetal(2003)Leptinstimulatestheoxidativeburstincontrolmono-cytesbutattenuatestheoxidativeburstinmonocytesfromHIV-infectedpatients.
ClinExpImmunol134(3):464–469782SeminImmunopathol(2012)34:771–78562.
GabayC,DreyerMG,PellegrinelliN,ChicheporticheR,MeierCA(2001)Leptindirectlyinducesthesecretionofinterleukin1receptorantagonistinhumanmonocytes.
JCEM86(2):783–79163.
MeierCA,ChicheporticheR,DreyerM,DayerJ-M(2003)IP-10,butnotRANTES,isupregulatedbyleptininmonocyticcells.
Cytokine21(1):43–4764.
BatraA,OkurB,GlaubenRetal(2010)Leptin:acriticalregulatorofCD4+T-cellpolarizationinvitroandinvivo.
Endo-crinology151(1):56–6265.
PadidarS,FarquharsonAJ,WilliamsLMetal(2011)Leptinup–regulatespro–inflammatorycytokinesindiscretecellswithinmousecolon.
JCellPhysiol226(8):2123–213066.
SitaramanS,LiuX,CharrierL,etal(2004)Colonicleptin:sourceofanovelpro-inflammatorycytokineinvolvedininflam-matoryboweldisease.
FASEBJ.
Availableat:http://www.
fasebj.
org/content/early/2004/03/31/fj.
03-0422fje.
Accessed15March201267.
ElKasmiKC,HolstJ,CoffreMetal(2006)GeneralnatureoftheSTAT3-activatedanti-inflammatoryresponse.
JImmunol177(11):7880–788868.
HruzP,DannSM,EckmannL(2010)STAT3anditsactivatorsinintestinaldefenseandmucosalhomeostasis.
CurrOpinGastro-enterol26(2):109–11569.
GoveME,RhodesDH,PiniMetal(2009)Roleofleptinreceptor-inducedSTAT3signalinginmodulationofintestinalandhepaticinflammationinmice.
JLeukocBiol85(3):491–49670.
ChenC,ChangY-C,LiuC-Letal(2007)LeptinInducesprolif-erationandanti-apoptosisinhumanhepatocarcinomacellsbyup-regulatingcyclinD1anddown-regulatingBaxviaajanuskinase2-linkedpathway.
EndocrRelatCancer14(2):513–52971.
OgunwobiO,BealesI(2007)Theanti-apoptoticandgrowthstimulatoryactionsofleptininhumancoloncancercellsinvolvesactivationofJNKmitogenactivatedproteinkinase,JAK2andPI3kinase/Akt.
IntJColorDis22(4):401–40972.
SukhotnikI,HelouH,LurieMetal(2007)Theeffectofleptinonintestinalrecoveryfollowingischemia-reperfusioninjuryinarat.
PediatrSurgInt23(5):473–47873.
SukhotnikI,VadaszZ,CoranAetal(2006)Effectofleptinonintestinalre-growthfollowingmassivesmallbowelresectioninrat.
PediatrSurgInt22(1):9–1574.
MattioliB,StrafaceE,QuarantaMG,GiordaniL,VioraM(2005)LeptinpromotesdifferentiationandsurvivalofhumandendriticcellsandlicensesthemforTh1priming.
JImmunol174(11):6820–682875.
LamQLK,LiuS,CaoX,LuL(2006)Involvementofleptinsignalinginthesurvivalandmaturationofbonemarrow–deriveddendriticcells.
EurJImmunol36(12):3118–313076.
MattioliB,GiordaniL,QuarantaMG,VioraM(2009)Leptinexertsananti-apoptoticeffectonhumandendriticcellsviathePI3K-Aktsignalingpathway.
FEBSLett583(7):1102–110677.
MarieCS,VerkerkeHP,PaulSN,MackeyAJ,PetriWA(2012).
LeptinprotectshostcellsfromEntamoebahistolyticacytotoxic-itybyaSTAT-3dependentmechanism.
InfectImmun.
Availableat:http://iai.
asm.
org/content/early/2012/02/06/IAI.
06140-11.
Accessed24February201278.
WankeC,ButlerT,IslamM(1988)EpidemiologicandclinicalfeaturesofinvasiveamebiasisinBangladesh:acase–controlcomparisonwithotherdiarrhealdiseasesandpostmortemfind-ings.
AmJTropMedHyg38(2):335–34179.
WallaceJL,VongL,DharmaniP,SrivastavaV,ChadeeK(2011)Muc-2-deficientmicedisplayasex-specific,COX-2-relatedimpair-mentofgastricmucosalrepair.
AmJPathol178(3):1126–113380.
BergstromKSB,Kissoon-SinghV,GibsonDLetal(2010)Muc2protectsagainstlethalinfectiouscolitisbydisassociatingpatho-genicandcommensalbacteriafromthecolonicmucosa.
PLoSPathog6(5):e100090281.
ChadeeK,PetriWA,InnesDJ,RavdinJI(1987)RatandhumancolonicmucinsbindtoandinhibitadherencelectinofEntamoebahistolytica.
JClinInvest80(5):1245–125482.
MoncadaDM,KammanadimintiSJ,ChadeeK(2003)MucinandToll-likereceptorsinhostdefenseagainstintestinalparasites.
TrendsParasitol19(7):305–31183.
LidellME,MoncadaDM,ChadeeK,HanssonGC(2006)Ent-amoebahistolyticacysteineproteasescleavetheMUC2mucininitsC-terminaldomainanddissolvetheprotectivecolonicmucusgel.
ProcNatlAcadSciUSA103(24):9298–930384.
BansalD,AveP,KerneisSetal(2009)Anex-vivohumanintestinalmodeltostudyEntamoebahistolyticapathogenesis.
PLoSNeglTropDis3(11):e55185.
BosHJ,vandeGriendRJ(1977)VirulenceandtoxicityofaxenicEntamoebahistolytica.
Nature265(5592):341–34386.
BhattacharyaA,GhildyalR,PrasadJ,BhattacharyaS,DiamondLS(1992)ModulationofasurfaceantigenofEntamoebahisto-lyticainresponsetobacteria.
InfectImmun60(4):1711–171387.
RaniR,MurthyRS,BhattacharyaSetal(2006)Changesinbacte-rialprofileduringamebiasis:demonstrationofanaerobicbacteriainALApussamples.
AmJTropMedHyg75(5):880–88588.
CrawfordPA,CrowleyJR,SambandamNetal(2009)Regulationofmyocardialketonebodymetabolismbythegutmicrobiotaduringnutrientdeprivation.
ProcNatlAcadSciUSA106:11276–1128189.
JumpertzR,LeDS,TurnbaughPJetal(2011)Energy-balancestudiesrevealassociationsbetweengutmicrobes,caloricload,andnutrientabsorptioninhumans.
AmJClinNutr94(1):58–6590.
KauAL,AhernPP,GriffinNW,GoodmanAL,GordonJI(2011)Humannutrition,thegutmicrobiome,andimmunesystem:envi-sioningthefuture.
Nature474(7351):327–33691.
GuptaSS,MohammedMH,GhoshTSetal(2011)Metagenomeofthegutofamalnourishedchild.
GutPathog3:792.
TovyA,HertzR,Siman-TovRetal(2011)GlucosestarvationboostsEntamoebahistolyticavirulence.
PLoSNeglTropDis5(8):e124793.
Serrano-LunaJ,Gutiérrez-MezaM,Mejía-ZepedaRetal(2010)Effectofphosphatidylcholine-cholesterolliposomesonEntamoe-bahistolyticavirulence.
CanJMicrobiol56(12):987–99594.
LeeJ,ParkSJ,YongTS(2008)EffectofirononadherenceandcytotoxicityofEntamoebahistolyticatoCHOcellmonolayers.
KoreanJParasitol46(1):37–4095.
CoboER,HeC,HirataKetal(2012)Entamoebahistolyticainducesintestinalcathelicidinsbutisresistanttocathelicidin-mediatedkilling.
InfectImmun80(1):143–14996.
GranlundAVB,BeisvagV,TorpSHetal(2011)ActivationofREGfamilyproteinsincolitis.
ScandJGastroenterol46(11):1316–132397.
PetersonKM,GuoX,ElkahlounAGetal(2011)TheexpressionofREG1AandREG1Bisincreasedduringacuteamebiccolitis.
ParasitolInt60(3):296–30098.
MukherjeeS,VaishnavaS,HooperLV(2008)Multi-layeredregulationofintestinalantimicrobialdefense.
CellMolLifeSci65(19):3019–302799.
KollsJK,McCrayPB,ChanYR(2008)Cytokine-mediatedregu-lationofantimicrobialproteins.
NatRevImmunol8(11):829–835100.
KammanadimintiSJ,MannBJ,DutilL,ChadeeK(2004)Reg-ulationofToll-likereceptor-2expressionbytheGal-lectinofEntamoebahistolytica.
FASEBJ18(1):155–157101.
BeckerSM,ChoK-N,GuoXetal(2010)EpithelialcellapoptosisfacilitatesEntamoebahistolyticainfectioninthegut.
AmJPathol176(3):1316–1322102.
EckmannL,ReedSL,SmithJR,KagnoffMF(1995)Entamoebahistolyticatrophozoitesinduceaninflammatorycytokinere-sponsebyculturedhumancellsthroughtheparacrineactionofcytolyticallyreleasedinterleukin-1alpha.
JClinInvest96(3):1269–1279SeminImmunopathol(2012)34:771–785783103.
HouY,MortimerL,ChadeeK(2010)Entamoebahistolyticacysteineproteinase5bindsintegrinoncoloniccellsandstimu-latesNFκB-mediatedpro-inflammatoryresponses.
JBiolChem285(46):35497–35504104.
IvoryCPA,PrystajeckyM,JobinC,ChadeeK(2008)Toll-likereceptor9-dependentmacrophageactivationbyEntamoebahis-tolyticaDNA.
InfectImmun76(1):289–297105.
SeydelKB,LiE,SwansonPE,StanleySL(1997)Humanintes-tinalepithelialcellsproduceproinflammatorycytokinesinre-sponsetoinfectioninaSCIDmouse–humanintestinalxenograftmodelofamebiasis.
InfectImmun65(5):1631–1639106.
KammanadimintiSJ,ChadeeK(2006)SuppressionofNF-κBactivationbyEntamoebahistolyticainintestinalepithelialcellsismediatedbyheatshockprotein27.
JBiolChem281(36):26112–26120107.
SalataRA,AhmedP,RavdinJI(1989)ChemoattractantactivityofEntamoebahistolyticaforhumanpolymorphonuclearneutro-phils.
JParasitol75(4):644–646108.
ChadeeK,MoreauF,MeerovitchE(1987)Entamoebahistoly-tica:chemoattractantactivityforgerbilneutrophilsinvivoandinvitro.
ExpParasitol64(1):12–23109.
YuY,ChadeeK(1997)Entamoebahistolyticastimulatesinter-leukin8fromhumancolonicepithelialcellswithoutparasite–enterocytecontact.
Gastroenterology112(5):1536–1547110.
ReedS,EmberJ,HerdmanDetal(1995)TheextracellularneutralcysteineproteinaseofEntamoebahistolyticadegradesanaphylatoxinsC3aandC5a.
JImmunol155(1):266–274111.
SalataRA,RavdinJI(1986)TheinteractionofhumanneutrophilsandEntamoebahistolyticaincreasescytopathogenicityforlivercellmonolayers.
JInfectDis154(1):19–26112.
GuerrantRL,BrushJ,RavdinJI,SullivanJA,MandellGL(1981)InteractionbetweenEntamoebahistolyticaandhumanpolymorphonuclearneutrophils.
JInfectDis143(1):83–93113.
MontecuccoF,BianchiG,GnerrePetal(2006)Inductionofneutrophilchemotaxisbyleptin.
AnnNYAcadSci1069(1):463–471114.
Caldefie-ChezetF,PoulinA,TridonA,SionB,VassonM-P(2001)Leptin:apotentialregulatorofpolymorphonuclearneu-trophilbactericidalactionJLeukocBiol69(3):414–418115.
NathanC(2006)Neutrophilsandimmunity:challengesandopportunities.
NatRevImmunol6(3):173–182116.
BrinkmannV,ReichardU,GoosmannCetal(2004)Neutrophilextracellulartrapskillbacteria.
Science303(5663):1532–1535117.
Rivero-NavaL,Aguirre-GarcaJ,Shibayama-SalasMetal(2002)Entamoebahistolytica:acutegranulomatousintestinallesionsinnormalandneutrophil-depletedmice.
ExpParasitol101(4):183–192118.
Jarillo-LunaR,Campos-RodrguezR,TsutsumiV(2002)Ent-amoebahistolytica:immunohistochemicalstudyofhepaticam-oebiasisinmouse.
Neutrophilsandnitricoxideaspossiblefactorsofresistance.
ExpParasitol101(1):40–56119.
RigothierM-C,KhunH,TavaresPetal(2002)FateofEntamoe-bahistolyticaduringestablishmentofamoebicliverabscessanalyzedbyquantitativeradioimagingandhistology.
InfectImmun70(6):3208–3215120.
SeydelKB,ZhangT,StanleySL(1997)Neutrophilsplayacriticalroleinearlyresistancetoamebicliverabscessesinseverecombinedimmunodeficientmice.
InfectImmun65(9):3951–3953121.
ChoiM-H,SajedD,PooleLetal(2005)AnunusualsurfaceperoxiredoxinprotectsinvasiveEntamoebahistolyticafromoxi-dantattack.
MolBiochemParasitol143(1):80–89122.
BruchhausI,RichterS,TannichE(1997)Removalofhydrogenperoxidebythe29kDaproteinofEntamoebahistolytica.
Bio-chemJ326(Pt3):785–789123.
Maldonado–BernalC,KirschningCJ,RosensteinYetal(2005)TheinnateimmuneresponsetoEntamoebahistolyticalipopeptidophosphoglycanismediatedbytoll–likereceptors2and4.
ParasiteImmunology27(4):127–137124.
SeydelKB,SmithSJ,StanleySL(2000)Innateimmunitytoamebicliverabscessisdependentongammainterferonandnitricoxideinamurinemodelofdisease.
InfectImmun68(1):400–402125.
Siman-TovR,AnkriS(2003)Nitricoxideinhibitscysteineproteinasesandalcoholdehydrogenase2ofEntamoebahistoly-tica.
ParasitolRes89(2):146–149126.
ElnekaveK,Siman–TovR,AnkriS(2003)ConsumptionofL–argininemediatedbyEntamoebahistolyticaL–arginase(EhArg)inhibitsamoebicidalactivityandnitricoxideproductionbyacti-vatedmacrophages.
ParasiteImmunology25(11–12):597–608127.
WangW,KellerK,ChadeeK(1994)Entamoebahistolyticamodulatesthenitricoxidesynthasegeneandnitricoxideproduc-tionbymacrophagesforcytotoxicityagainstamoebaeandtu-mourcells.
Immunology83(4):601–610128.
LinJY,KellerK,ChadeeK(1993)Entamoebahistolyticapro-teinsmodulatetherespiratoryburstpotentialbymurinemacro-phages.
Immunology78(2):291–297129.
SeguinR,KellerK,ChadeeK(1995)Entamoebahistolyticastimulatestheunstabletranscriptionofc-fosandtumournecrosisfactor-alphamRNAbyproteinkinaseCsignaltransductioninmacrophages.
Immunology86(1):49–57130.
DeyI,KellerK,BelleyA,ChadeeK(2003)Identificationandcharacterizationofacyclooxygenase-likeenzymefromEntamoe-bahistolytica.
ProcNatlAcadSciUSA100(23):13561–13566131.
DeyI,ChadeeK(2008)ProstaglandinE2producedbyEntamoe-bahistolyticabindstoEP4receptorsandstimulatesinterleukin-8productioninhumancoloniccells.
InfectImmun76(11):5158–5163132.
Rico-RosilloG,Díaz-GuerraO,KretschmerRR(1990)[Mono-cytelocomotioninhibitingfactor(MLIF)producedbyE.
histo-lyticainducesanincreaseofcAMPinhumanmonocytes].
ArchInvestMed(Mex)21(Suppl1):245–247133.
KretschmerR,CastroEM,ArellanoJ,PachecoMG(1986)InvitrostudiesontheinteractionofhumanmonocytesandthemonocytelocomotioninhibitoryfactorproducedbyE.
histoly-tica.
ArchInvestMed(Mex)17(Suppl1):243–246134.
LotterH,JacobsT,GaworskiI,TannichE(2006)Sexualdimor-phisminthecontrolofamebicliverabscessinamousemodelofdisease.
InfectImmun74(1):118–124135.
GordonS,MartinezFO(2010)Alternativeactivationofmacro-phages:mechanismandfunctions.
Immunity32(5):593–604136.
CohenNR,GargS,BrennerMB(2009).
Chapter1antigenpresen-tationbyCD1:lipids,Tcells,andNKTcellsinmicrobialimmunity.
In:AdvancesinImmunology.
Volume102.
AcademicPress,pp.
1–94.
Availableat:http://www.
sciencedirect.
com/science/article/pii/S0065277609012012.
Accessed26February2012137.
TsutsumiV,Mena-LopezR,Anaya-VelazquezF,Martinez-PalomoA(1984)Cellularbasesofexperimentalamebicliverabscessformation.
AmJPathol117(1):81–91138.
KimKH,ShinCO,ImK(1993)Naturalkillercellactivityinmiceinfectedwithfree-livingamoebawithreferencetotheirpathogenicity.
TheKoreanJournalofParasitology31(3):239139.
FusakioME,MohammedJP,LaumonnierYetal(2011)C5aregulatesNKTandNKcellfunctionsinsepsis.
JImmunol187(11):5805–5812140.
GodfreyDI,MacDonaldHR,KronenbergM,SmythMJ,KaerLV(2004)NKTcells:what'sinanameNatRevImmunol4(3):231–237141.
LotterH,González-RoldánN,LindnerBetal(2009)NaturalkillerTcellsactivatedbyalipopeptidophosphoglycanfromEnt-amoebahistolyticaarecriticallyimportanttocontrolamebicliverabscess.
PLoSPathog5(5):e1000434142.
DunkelbergerJR,SongW-C(2009)Complementanditsroleininnateandadaptiveimmuneresponses.
CellRes20(1):34–50784SeminImmunopathol(2012)34:771–785143.
Ortiz-OrtizL,CapinR,CapinNR,SepúlvedaB,ZamaconaG(1978)ActivationofthealternativepathwayofcomplementbyEntamoebahistolytica.
ClinExpImmunol34(1):10–18144.
ReedS,KeeneW,McKerrowJ,GigliI(1989)CleavageofC3byaneutralcysteineproteinaseofEntamoebahistolytica.
JImmu-nol143(1):189–195145.
ReedSL,GigliI(1990)Lysisofcomplement-sensitiveEntamoe-bahistolyticabyactivatedterminalcomplementcomponents.
Initiationofcomplementactivationbyanextracellularneutralcysteineproteinase.
JClinInvest86(6):1815–1822146.
ReedS,CurdJ,GigliI,GillinF,BraudeA(1986)ActivationofcomplementbypathogenicandnonpathogenicEntamoebahisto-lytica.
JImmunol136(6):2265–2270147.
Flores-RomoL,TsutsumiV,Estrada-GarcíaTetal(1994)CD59(protectin)molecule,resistancetocomplement,andvirulenceofEntamoebahistolytica.
TransRSocTropMedHyg88(1):116–117148.
BragaLL,NinomiyaH,McCoyJJetal(1992)Inhibitionofthecomplementmembraneattackcomplexbythegalactose-specificadhesionofEntamoebahistolytica.
JClinInvest90(3):1131–1137149.
WeberC,BlazquezS,MarionSetal(2008)Bioinformaticsandfunctionalanalysisofanentamoebahistolyticamannosyltrans-ferasenecessaryforparasitecomplementresistanceandhepaticalinfection.
PLoSNeglTropDis2(2):e165150.
Ventura-JuárezJ,Campos-RodríguezR,Jarillo-LunaRAetal(2008)TrophozoitesofEntamoebahistolyticaexpressaCD59-likemoleculeinhumancolon.
ParasitolRes104(4):821–826151.
BhattacharyaA,AryaR,ClarkCG,AckersJP(2000)Absenceoflipophosphoglycan-likeglycoconjugatesinEntamoebadispar.
Parasitology120(01):31–35152.
SacksD,SherA(2002)Evasionofinnateimmunitybyparasiticprotozoa.
NatImmunol3(11):1041–1047153.
Acuna-SotoR,MaguireJH,WirthDF(2000)Genderdistributioninasymptomaticandinvasiveamebiasis.
AmJGastroenterol95(5):1277–1283154.
SnowM,ChenM,GuoJ,AtkinsonJ,StanleySL(2008)Differ-encesincomplement-mediatedkillingofEntamoebahistolyticabetweenmenandwomen—anexplanationfortheincreasedsusceptibilityofmentoinvasiveamebiasisAmJTropMedHyg78(6):922–923SeminImmunopathol(2012)34:771–785785
- characterized8090lu.com相关文档
- 工商会8090lu.com
- 地址8090lu.com
- 济南市8090lu.com
- 辛辛那提8090lu.com
- "ESMAppendixII:Brothersgeochemistrydata"
- 11.278090lu.com
hostyun评测香港原生IPVPS
hostyun新上了香港cloudie机房的香港原生IP的VPS,写的是默认接入200Mbps带宽(共享),基于KVM虚拟,纯SSD RAID10,三网直连,混合超售的CN2网络,商家对VPS的I/O有大致100MB/S的限制。由于是原生香港IP,所以这个VPS还是有一定的看头的,这里给大家弄个测评,数据仅供参考!9折优惠码:hostyun,循环优惠内存CPUSSD流量带宽价格购买1G1核10G3...
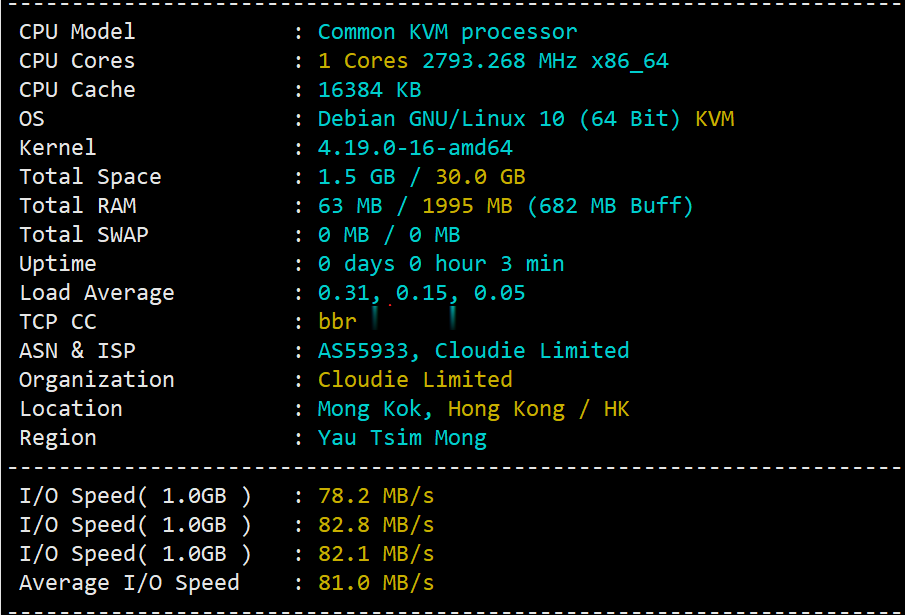
buyvm美国大硬盘VPS,1Gbps带宽不限流量
buyvm正式对外开卖第四个数据中心“迈阿密”的块存储服务,和前面拉斯维加斯、纽约、卢森堡一样,依旧是每256G硬盘仅需1.25美元/月,最大支持10T硬盘。配合buyvm自己的VPS,1Gbps带宽、不限流量,在vps上挂载块存储之后就可以用来做数据备份、文件下载、刷BT等一系列工作。官方网站:https://buyvm.net支持信用卡、PayPal、支付宝付款,支付宝付款用的是加元汇率,貌似...
ZJI(月付480元),香港阿里云专线服务器
ZJI是成立于2011年原Wordpress圈知名主机商—维翔主机,2018年9月更名为ZJI,主要提供香港、日本、美国独立服务器(自营/数据中心直营)租用及VDS、虚拟主机空间、域名注册业务。本月商家针对香港阿里云线路独立服务器提供月付立减270-400元优惠码,优惠后香港独立服务器(阿里云专线)E3或者E5 CPU,SSD硬盘,最低每月仅480元起。阿里一型CPU:Intel E5-2630L...
8090lu.com为你推荐
-
brandoff国际大牌包包都有哪些呐?美国互联网瘫痪如果全球网络瘫痪3分钟会造成多少损失openeuleropen opening opens opened有什么区别sonicchat深圳哪里有卖汽车模型?关键字关键词编故事原代码源代码是什么rawtoolsTF卡被写保护了怎么办?郭泊雄郭佰雄最后一次出现是什么时候?www.7788dy.comwww.tom365.com这个免费的电影网站有毒吗?kb123.net股市里的STAQ、NET市场是什么?