dependsjavmoo.com
javmoo.com 时间:2021-03-19 阅读:()
ISSN0030-400X,OpticsandSpectroscopy,2009,Vol.
106,No.
4,pp.
556–563.
PleiadesPublishing,Ltd.
,2009.
OriginalRussianTextD.
A.
Spasskii,V.
N.
Kolobanov,V.
V.
Mikhalin,L.
Yu.
Berezovskaya,L.
I.
Ivleva,I.
S.
Voronina,2009,publishedinOptikaiSpektroskopiya,2009,Vol.
106,No.
4,pp.
625–632.
556INTRODUCTIONMolybdenum-containingcrystalsarepromisingforcryogenicscintillationbolometers.
The100Moisotopeisapotential(andmostpromising)sourceofdoublenon-neutrinoβ-decay0ν2β[1–4].
Thepossibilityofimplementinga0ν2βsourceanddetectingthisdecayinthesamematerialisveryattractivebecauseitallowsonetosignicantlyincreasethedetectionefciency.
Themainrequirementsforapotentialscintillatorareahighspeciclightyieldwithshortdecaytimes(lessthan10–4s),theabsenceofradioactiveisotopesfortheotherelementsinthecrystalcomposition,lowdensity,andsmalleffectiveatomicnumberZ(whichisneces-sarytoreducebackground).
Untilnow,themostatten-tionhasbeenpaidtothescintillationpropertiesofmolybdateswithascheelite-typestructure:AMoO4(A=Ca,Cd,Sr,Ba,Pb).
However,allofthecrystalsstudiedhaveanumberofdrawbacks,i.
e.
,lowlumines-cenceintensity,evenatlowtemperatures(BaMoO4);largeZ(PbMoO4);orthepresenceofradioactivecationisotopes(CdMoO4,PbMoO4).
Currently,CMoO4crystalsareconsideredasthemostappropriate.
How-ever,the48Caisotopeisasourceofthe2ν2βdecay,whichformsanunremovablebackgroundindetection.
Themagnesiummolybdate(MgMoO4)canbeanalter-nativetocrystalswithscheelitestructures.
Theabsenceoflong-livedradioactiveisotopesofmagnesium,aswellasthelowdensityofMgMoO4(4.
04g/cm3)areadvantagesincomparisonwithscheelitecrystals.
MgMoO4luminescencewasobservedforthefirsttimein[5];however,amoredetailedinvestigationoftheluminescenceandopticalpropertiesofthiscompoundhavebeenperformedonlyrecently[6–8].
Inthispaper,wereporttheresultsofstudyingtheluminescencecharacteristicsofMgMoO4crystalsgrownunderdifferentconditions.
Thereectionspec-traofMgMoO4weremeasuredforthersttimetakingintoaccountthecrystalstructureanisotropy.
Recently,muchattentionhasbeenfocusedoncharge-transferluminescence,whichwasobservedinanumberofoxy-gen-containingcompoundsdopedwithYb3+ions[9].
Therefore,wehavestudiedtheluminescencepropertiesofMgMoO4:Ybcrystals.
EXPERIMENTALTheluminescenceVUV-excitationspectra,trans-missionspectranearthefundamentalabsorptionedge,andluminescenceexcitationandreectionspectraintheenergyrange3.
7–25eVweremeasuredontheSuperlumisystem(DEZY,Hamburg)[10]attempera-turesof8–70Kandat300K.
TheluminescencespectraweremeasuredusinganARCSpectraProSP-308monochromator(inthespectrographmode)andnor-malizedtothespectralsensitivityfunctionofthedetectingcomplex.
Plane-parallelsurfacesoffreshcleavageswereanalyzed.
ThecrystalsunderstudyweregrownbytheCzo-chralskimethodinplatinumcruciblesinair.
SampleMgMoO4no.
1wasgrownfromastoichiometricmeltofMgOandMoO3ofhigh-puritygrade.
Thechargecom-ponentsweredriedandmixedinastoichiometricpro-portion,thenheatedinaplatinumcrucibleat700or930°Cfor6h.
X-raydiffractionanalysisshowedthatCONDENSED-MATTERSPECTROSCOPYLuminescencePeculiaritiesandOpticalPropertiesofMgMoO4andMgMoO4:YbCrystalsD.
A.
Spasskiia,V.
N.
Kolobanovb,V.
V.
Mikhalinb,L.
Yu.
Berezovskayac,L.
I.
Ivlevac,andI.
S.
VoroninacaSkobeltsynInstituteofNuclearPhysics,MoscowStateUniversity,Moscow,119992RussiabFacultyofPhysics,MoscowStateUniversity,Moscow,119991RussiacProkhorovGeneralPhysicsInstitute,RussianAcademyofSciences,Moscow,119991Russiae-mail:deris2002@mail.
ruReceivedOctober20,2008Abstract—Magnesiummolybdateisconsideredasapromisingmaterialforcryogenicscintillationbolometers.
TheluminescencepropertiesofMgMoO4havebeeninvestigatedonsinglecrystalsgrownfrommeltsofsto-ichiometricandnonstoichiometriccompositionsandoncrystalsdopedwithYb3+ions.
TheiropticalpropertiesareinterpretedtakingintoaccounttheanisotropyoftheMgMoO4crystalstructure.
PACSnumbers:78.
55.
-m,78.
40.
-qDOI:10.
1134/S0030400X09040171OPTICSANDSPECTROSCOPYVol.
106No.
42009LUMINESCENCEPECULIARITIESANDOPTICALPROPERTIES557synthesisat700°CleadstotheformationofbothMgMoO4andMg2Mo3O11phases.
At930°C,onlytheβ-MgMoO4phaseissynthesized.
Themeltingtemper-atureofMgMoO4is1320°C.
Thepullingraterangedfrom1to3mm/h.
SampleMgMoO4no.
2wasgrownfromanonstoichiometricmeltwithadecitofMoO3(3wt%).
SampleMgMoO4no.
3wasobtainedfromastoichiometricmeltwithadditionof5wt%Yb2O3.
β-MgMoO4isabiaxialanisotropiccrystalwiththeunit-cellparametersa=10.
273,b=9.
288,c=7.
025,andβ=106.
96°;itbelongstothespacegroupC2/m[11].
ItisknownthatthereflectionofanisotropicmolybdatecrystalsdependsontherelativeorientationoftheircrystallographicaxesandtheelectricvectorEoftheincidentpolarizedsynchrotronradiation.
There-fore,thesamplesunderstudywereorientedalongthecrystallographicaxesbyelectronbackscattereddiffrac-tion(EBSD)usingaCRYSTALattachment(OxfordInstrumentsINCASystem)toaJSM5910-10LV(Jeol)scanningelectronmicroscope.
ItwasshownthattheMgMoO4cleavageplanebelongsto{9110}crystallo-graphicplanes.
Thus,thecrystallographicaxiscliesintheMgMoO4cleavageplane.
Duringmeasurements,thesampleswereorientedsoastomakethecaxisbeeitherparallel(c||E)orperpendicular(c⊥E)totheprojectionoftheincidentradiationvectorEontothesampleplane.
RESULTSANDDISCUSSIONAbsorptionSpectraofMgMoO4TheabsorptionspectraatT=10and300KareshowninFig.
1.
Atatemperatureof10K,theabsorp-tioncoefcientbeginstosignicantlyincreaseat3.
9eV.
Withanincreaseintemperatureto300K,theabsorptionedgeundergoesaredshiftandbecomesat-ter.
SuchbehaviorischaracteristicofthefundamentalabsorptionedgeofdielectricsandcanbedescribedbytheUrbachformula[12]whereσistheslope,Tistemperature,kBistheBoltz-mannconstant,andα0andE0arethecoordinatesoftheintersectionpointsoftheUrbachedgesatdifferenttem-peratures,extrapolatedtolargeabsorptioncoefcients.
ApproximationoftheabsorptionspectrabytheUrbachformulagavethefollowingvaluesofσandE0forMgMoO4:σ=0.
32andE0=4.
49eVatT=300Kandσ=0.
02andE0=4.
57eVatT=10K.
NotethatE0isclosetotheexcitonpeakpositionintheabsorptionspectrumandthattheslopeparameterσdependsontemperatureandcangiveinformationaboutthepossi-bilityofexcitonself-trappinginthesecompounds[12].
ThereectionspectraofMgMoO4samplesmea-suredinthefundamentalabsorptionrangewererecal-culatedintoabsorptionspectra,becausethelatteryieldmoreexactenergiesofinterbandelectronictransitions.
Wealsocalculatedtheenergydependencesoftheimag-inaryandrealpartsofthepermittivityandenergy-lossfunction.
TherecalculationwasperformedaccordingtotheKramers–KronigrelationsusingfastFouriertrans-form[13].
Thesecalculationsrequireinformationaboutthebehaviorofthereectioncoefcientintheentirefrequencyrange.
Theexperimentalreectionspectraweremeasuredatenergiesfrom4to25eV.
Thereec-αE()α0σE0E–kBT-–,exp=0.
0203.
8Intensity,arb.
unitsPhotonenergy,eV4.
04.
20.
041234110α,cm–1Fig.
1.
(1,2)AbsorptionspectraofMgMoO4no.
1atT=(1)300and(2)10KandtheluminescenceexcitationspectrameasuredatT=10Kforsamples(3)MgMoO4no.
1(Elum=2.
3eV)and(4)MgMoO4no.
2(Elum=2.
0eV).
558OPTICSANDSPECTROSCOPYVol.
106No.
42009SPASSKIIetal.
tioncoefcientRwasextrapolatedintheenergyrangeE>25eVaccordingtothelawR~E–4.
Inthelow-energyregion,weappliedtheself-consistentprocedureforextrapolatingthereectioncoefcient,whichusestherefractiveindexasaparameter.
ThecriterionforrecalculationcorrectnesswasthevalidityoftheKram-ers–Kronigrelationsforthecalculatedpermittivity,aswellastheconvergenceofthecalculatedfourtypesofthesumrule,obtainedfromtheimaginarypartofthepermittivityandtheenergy-lossfunction.
TheobtainedabsorptionandintrinsicluminescenceexcitationspectraareshowninFig.
2.
Dependingonthesampleorientation,uptoeightabsorptionpeaksareobservedintheenergyrange4–25eV;theirenergypositionsatT=10KarelistedinTable1.
TheenergiesoftheabsorptionpeaksexceedsthevalueE0givenbytheUrbachformula.
Indeed,theabsorptionspectrumlacksanypronouncedstructureintheenergyrange4.
5–4.
6eV,whichcouldbeassignedtotheformationofanexcitonatthefundamentalabsorptionedge.
Theabsenceofexcitonischaracteris-ticofmolybdateswithscheelitestructure,forexample,CaMoO4andSrMoO4,forwhichthelow-energyreflec-tionpeakisdeterminedbyinterbandtransitions[14,15].
However,onecannotexcludethattheexcitonpeakishiddenduetothepresenceofstructuraldefectsintheMgMoO4samplesunderstudy.
Forexample,for0.
506Intensity,arb.
unitsPhotonenergy,eV481510202512*6(b)0.
5*10601.
0*1060.
5012*6(a)0.
5*10601.
0*1061.
01.
5*106α,cm–1Fig.
2.
(1)AbsorptionspectracalculatedaccordingtotheKramers–Kronigrelationsfromtheexperimentalreectionspectraand(2)intrinsicluminescenceexcitationspectra(Elum=2.
3eV)foraMgMoO4crystalwiththeorientations(a)c||Eand(b)c⊥E.
Thearrowsindicatethemostpronouncedantibaticpeculiaritiesintheabsorptionandluminescenceexcitationspectra.
Table1.
AbsorptionpeakenergiesforMgMoO4atdifferentorientationsofthecrystallographicaxiscwithrespecttotheprojectionoftheelectricvectorEofsynchrotronradiationincidentonthesamplesurface(T=10K)CrystalorientationAbsorptionpeakenergies,eV123456789c||E5.
15.
86.
256.
99.
110.
614.
117.
0~21c⊥E5.
25.
75–6.
89.
111.
213.
916.
5~21OPTICSANDSPECTROSCOPYVol.
106No.
42009LUMINESCENCEPECULIARITIESANDOPTICALPROPERTIES559LuAlO3,reliableinformationabouttheexistenceofanexcitonpeakatthefundamentalabsorptionedgecouldbeobtainedonlybymeasuringthereectionfromthesurfaceofsingle-crystallmshavinglessstructuraldefectsincomparisonwithbulksinglecrystals[16].
Apparently,thelow-energyabsorptionpeakintheMgMoO4samplesunderstudyisduetotheinterbandelectronictransitions.
Thepositionoftherst-peakmaximumintheabsorptionspectrumyieldsthefollow-ingestimationfromabovefortheMgMoO4bandgap:Eg30K,theexperimentaltemperaturedepen-denceoftheluminescenceintensityforMgMoO4no.
1differsfromthatcalculatedfromtheMottformula.
Thereasonforthisisthatanadditionalbandarisesintheluminescencespectrumasaresultofthesignicantdecreaseintheintrinsicluminescenceintensity(approximatelybytwoordersofmagnitude).
Indeed,anincreaseintemperatureto30Kshiftsthebandpeakfrom2.
3to2.
0eV;thebandbecomesnonelementaryandcanberepresentedasasuperpositionoftwoele-mentaryluminescencebandspeakingat2.
3and1.
9eV.
Forsampleno.
2(grownfromachargewithadecitofMoO3),thenonelementaryluminescenceband,whichcanalsobedecomposedintoGaussiancomponents,isobservedevenatT=10K.
Theresultsofdecomposi-tionintoGaussiancomponentsforsamplesno.
1andno.
2arelistedinTable3.
Theexcitationspectrumoftheluminescencebandat1.
9eVnearthefundamentalabsorptionedge(Fig.
1)ischaracterizedbytheformationofanadditionalexci-tationbandat4.
0eV.
At10K,thisbandisinthetrans-parencyrangeofMgMoO4.
Therelativeweightofthelow-energyluminescencebandincreasesuponlumi-nescenceexcitationat4.
0eV.
Wecanconcludethattheluminescencebandat1.
9eVisduetothedefectscausedbythecrystaldeviationfromstoichiometry.
Twooverlappingluminescencebandsofcompara-bleintensitywereobservedfornominallypureMgMoO4atT=9K[6].
Thebandpeaksobtainedinthisstudyarered-shiftedby~0.
2eVwithrespecttothedataof[6].
Ourconclusionaboutthedefectnatureofthe1.
9-eVluminescencebandisinagreementwithoneofthesuggestionsmadein[6]aboutthenatureofthelow-energybandat~2.
1eV.
Notethattheabsenceofasignicantcontributionofthelow-energydefectbandatT=10KforsampleMgMoO4no.
1indicatesitshighstructuralquality.
Theluminescencespectrumofmagnesiummolyb-datedopedwithytterbiumions(sampleno.
3)contains,alongwiththeintrinsicluminescenceband,additionalnarrowluminescencebandsat1.
21,1.
24,and1.
27eV,whichcorrespondstothef–ftransitionsinYb3+ions[9].
Thecharge-transferluminescence,characteristicofsomecomplexoxides(garnets,perovskites,borates)dopedwithYb3+,wasnotobservedinMgMoO4:Yb.
Notethattheintrinsicluminescenceintensityforsamplesno.
2andno.
3ismuchlowerthanforsampleno.
1.
Apparently,thisisduetotheformationofcom-petingluminescencecenters,whicharerelatedtothepresenceofbothstructuraldefectsandimpuritycentersinthenonstoichiometric(no.
2)anddoped(no.
3)sam-ples.
Theluminescenceintensityofmagnesiummolyb-dateandsomeothermolybdatesandtungstatesatlowtemperatures(T=10K)wascomparedunderthesameexperimentalconditions.
Toexcludetheenergylossduetothethermalizationofchargecarriers,theexcita-tionenergyforeachcrystalwaschosentocorrespondtothefundamentalabsorptionedge.
Thisapproachmakesitpossibletoshowthepotentialspeciclightyieldofacrystalwhenthecompetingchannelsofnon-radiativeenergyrelaxationaresuppressed.
Compari-sonwasperformedforMgMoO4no.
1,ZnMoO4,BaWO4,andCdWO4samples.
MgMoO4,ZnMoO4,andBaWO4singlecrystalsweregrownbytheCzo-chralskimethodattheGeneralPhysicsInstitute,Rus-sianAcademyofSciences.
TheCdWO4samplewaspreparedfroma1-cm3crystalwithaspeciclightyieldTable3.
EnergiesandFWHMsoftheintrinsicluminescenceband(Emax1)andtheluminescencebandduetostructuralde-fects(Emax2)forMgMoO4no.
1andno.
2samplesatdifferenttemperaturesandexcitationenergiesCrystalT,KEex,eVEmax1,eVFWHM1,eVEmax2,eVFWHM2,eVMgMoO4no.
1105.
42.
310.
67––MgMoO4no.
1705.
42.
300.
691.
90(1.
21)0.
57MgMoO4no.
2105.
42.
310.
641.
92(0.
43)0.
53MgMoO4no.
2104.
02.
310.
651.
89(1.
28)0.
57Note:Theweightofalow-energyluminescencebandwithrespecttothehigh-energybandisgiveninparenthesesafterthemaximumvalue.
562OPTICSANDSPECTROSCOPYVol.
106No.
42009SPASSKIIetal.
of~20000photons/meVatT=300K;thecrystalwasgrownbytheCzochralskimethodattheScienticandTechnicalComplex"InstituteforSingleCrystals"(Kharkov,Ukraine).
ItisshownthattheluminescenceintensityofMgMoO4no.
1isanorderofmagnitudehigherthanthatofBaWO4butmuchlowerthantheluminescenceintensityofCdWO4andZnMoO4(byfactorsofabout40and10,respectively),whichcanbeaseriousproblemforpracticalapplicationofMgMoO4asascintillator.
LuminescenceExcitationSpectraTheintrinsicluminescenceexcitationspectraofMgMoO4no.
1areshowninFig.
2.
Thestructuralanisotropymanifestsitselfinthesespectra,whicharedifferentfordifferentorientationswithrespecttoE.
Apossiblereasonforthedifferenceintheexcitationspectraisthesurfacelossfactor.
Generally,theexcita-tionspectraaremodulatedbytheabsorptionspectra,duetowhichantibaticspecicfeaturesariseinthestructureoftheexcitationandabsorptionspectra[13].
Theantibaticspecicfeaturesthataremostpro-nouncedintheabsorptionandexcitationspectraareindicatedbyarrowsinFig.
2.
SincethepresenceandrelativeintensityoftheabsorptionpeaksinMgMoO4dependonthecrystalorientation,themodulationoftheexcitationspectraobtainedintheparallelandperpen-dicularorientationsofcandEwillbedifferent.
Notethatinthecaseofthec||Eorientationtheexci-tationspectrumcontainstwoadditionalpeaksat4.
05and4.
4eV.
Theyareabsentforthec⊥Eorientation,andtheirappearancecannotbeexplainedbythesurfacelossfactor,becausepronouncedabsorptionpeaksareabsentinthisenergyrange.
Atthesametime,theinten-sityoftheexcitationspectrumincreasessimultaneouslywiththeabsorptioncoefcient(Fig.
1,curves2,3).
Apparently,thepresenceofthesepeaksonlyatthesampleorientationc||Echaracterizestheanisotropyoftheenergybandlevelsnearthefundamentalabsorptionedge.
Thesignicant(byapproximatelyanorderofmag-nitude)decreaseintheluminescenceintensitywithanincreaseintheexcitationenergyfrom4.
5–5.
5to11–12eVindicatestheexcitontypeofenergytransfertointrinsicluminescencecentersinthepresenceofeffec-tivecompetingchannelofenergyrelaxation.
Anincreaseintheexcitationenergyincreasestheaveragedistancebetweenthefreeelectronandhole.
Intheabsenceofeffectivecompetingrelaxationchannel,theprobabilityforafreeelectronandholetoformanexci-tondependsweaklyontheexcitationenergyintherangefromthefundamentalabsorptionedgetothebeginningofphotonmultiplication.
Thisbehaviorwasobserved,forexample,fortheluminescenceexcitationspectrumofself-trappedexcitonsinundopedCdWO4[26].
Inthepresenceofacompetingrelaxationchannel,theprobabilityofexcitonformationdecreaseswithanincreaseintheaveragedistancebetweenanelectronandhole.
SinceonlyoneluminescencebandisobservedintheMgMoO4no.
1crystalatT=10K(Fig.
4,curve1),thecompetingchannelisnonradiative.
Atphotonenergiesabove12eVtheintensityofexcitationspectraincreasesduetotheformationoflow-energyelectron–holepairsasaresultofmultipli-cationofelectronicexcitations.
CONCLUSIONSTheluminescenceandopticalpropertiesofMgMoO4crystalsgrownfromstoichiometricandnon-stoichiometricmeltsandcrystalsdopedwithYb3+ionswereinvestigated.
Considerationofthestructuralanisotropymadeitpossibletoexplainthemainpecu-liaritiesoftheabsorptionspectraintheenergyrange4–25eV.
Thebandgapisestimatedtobeintherange4.
52eV.
Itisshownthattheintrinsiclumines-cenceat2.
3eViscausedbytheemissionofexcitonsself-trappedatMoO4complexes.
Theexistenceofaneffectivenonradiativechannelofenergyrelaxation,whichcompeteswithintrinsicluminescencecenters,isestablished.
Itisconcludedthattheluminescencebandat1.
9eVisduetothedefectsofthesamplecrystalstructure.
DopingofMgMoO4withytterbiumionsleadstotheoccurrenceofimpurityluminescencebandsintheIRregion;charge-transferluminescenceisnotobservedinthiscase.
Theintrinsicluminescenceinten-sityofMgMoO4at10KismuchlowerthanthatofCdWO4andZnMoO4;hence,applicationofthiscrystalasancryogenicscintillatorislimited.
ACKNOWLEDGMENTSThisstudywassupportedbygrantDFG436RUS113/437/0-3andtheRussianFoundationforBasicResearch,projectno.
06-02-16339.
WearegratefultoG.
ZimmererforpermittingexperimentsonSUPER-LUMIandtoG.
Striganyukforhishelpinmeasure-ments.
WearealsogratefultoD.
ShaturaforsupplyingtheX-raydiffractiondata,L.
Iskhakovaforthesampleorientation,andI.
TupitsinaandL.
Nagornayaforsup-plyingtheCdWO4sample.
REFERENCES1.
M.
Minowa,K.
Itakura,S.
Motiyama,andW.
Ootani,Nucl.
Instrum.
MethodsPhys.
Res.
,Sect.
A320,500(1992).
2.
A.
N.
Annenkov,O.
A.
Buzanov,F.
A.
Danevich,etal.
,Nucl.
Instrum.
MethodsPhys.
Res.
,Sect.
A584,334(2008).
3.
S.
Belogurov,V.
Kornoukhov,A.
Annenkov,etal.
,IEEETrans.
Nucl.
Sci.
52(4),1131(2005).
4.
S.
Pirro,S.
Capelli,M.
Pavan,etal.
,http://arxiv.
org/abs/nucl-ex/0510074v1.
OPTICSANDSPECTROSCOPYVol.
106No.
42009LUMINESCENCEPECULIARITIESANDOPTICALPROPERTIES5635.
M.
Amberg,J.
R.
Gijnter,H.
Schmalle,andG.
Blasse,J.
SolidStateChem.
77,162(1988).
6.
V.
B.
MikhailikandH.
Kraus,J.
Phys.
D:Appl.
Phys.
39,1181(2006).
7.
V.
B.
Mikhailik,H.
Kraus,M.
Itoh,etal.
,J.
Phys.
:Con-dens.
Matter17(46),7209(2005).
8.
I.
V.
Kitaeva,V.
N.
Kolobanov,V.
V.
Mikhailin,etal.
,inProc.
8thInt.
Conf.
onInorganicScintillatorsandTheirUseinScienticandIndustrialApplications,Alushta,Ukraine,2005,p.
44.
9.
I.
Kamenskikh,N.
Guerassimova,A.
Dujardin,etal.
,Opt.
Mater.
24,267(2003).
10.
G.
Zimmerer,Radiat.
Meas.
42(4–5),859(2007).
11.
V.
V.
Babakin,R.
F.
Klevtsova,andL.
A.
Gaponenko,Kristallograya27(1),38(1982)[Sov.
Phys.
Crystal-logr.
27,20(1982)].
12.
K.
S.
SongandR.
T.
Williams,Self-TrappedExcitons,2nded.
,SpringerSeriesinSolid-StateSciences(Springer,Berlin,1996),Vol.
105.
13.
V.
V.
MikhalinandA.
N.
Vasil'ev,IntroductiontoSolid-StateSpectroscopy(Izd-voMGU,Moscow,1987)[inRussian].
14.
R.
Grasser,E.
Pitt,G.
Zimmerer,etal.
,Phys.
StatusSolidiB69,359(1975).
15.
D.
Spassky,S.
Ivanov,I.
Kitaeva,etal.
,Phys.
StatusSolidiC2(1),65(2005).
16.
V.
Kolobanov,V.
Mikhailin,N.
Petrovnin,D.
Spassky,andYu.
Zorenko,Phys.
StatusSolidiB243(8),R60(2006).
17.
J.
A.
Rodriguez,J.
C.
Hanson,S.
Chaturvedi,etal.
,J.
Chem.
Phys.
112(2),935(2000).
18.
W.
Lotz,J.
Opt.
Soc.
Am.
60(2),206(1970).
19.
V.
N.
Kolobanov,I.
A.
Kamenskikh,V.
V.
Mikhailin,etal.
,Nucl.
Instrum.
MethodsPhys.
Res.
,Sect.
A486(1–2),496(2002).
20.
E.
G.
Reut,Izv.
Akad.
NaukSSSR,Ser.
Fiz.
49(10),2032(1985).
21.
Y.
Zhang,N.
A.
W.
Holzwarth,andR.
T.
Williams,Phys.
Rev.
B57(20),12738(1998).
22.
W.
VanLoo,Phys.
StatusSolidiA27,565(1975).
23.
N.
F.
Mott,Proc.
R.
Soc.
London,Ser.
A167,384(1938).
24.
N.
Saito,N.
Sonoyama,andT.
Sakata,Bull.
Chem.
Soc.
Jpn.
69,2191(1996).
25.
D.
Spassky,V.
Kolobanov,V.
Mikhailin,etal.
,Phys.
Sta-tusSolidi(inpress).
26.
O.
V.
Rzhevskaya,D.
A.
Spasski,V.
N.
Kolobanov,etal.
,Opt.
Spektrosk.
104(3),407(2008)[Opt.
Spec-trosc.
104,366(2008)].
TranslatedbyYu.
Sin'kov
106,No.
4,pp.
556–563.
PleiadesPublishing,Ltd.
,2009.
OriginalRussianTextD.
A.
Spasskii,V.
N.
Kolobanov,V.
V.
Mikhalin,L.
Yu.
Berezovskaya,L.
I.
Ivleva,I.
S.
Voronina,2009,publishedinOptikaiSpektroskopiya,2009,Vol.
106,No.
4,pp.
625–632.
556INTRODUCTIONMolybdenum-containingcrystalsarepromisingforcryogenicscintillationbolometers.
The100Moisotopeisapotential(andmostpromising)sourceofdoublenon-neutrinoβ-decay0ν2β[1–4].
Thepossibilityofimplementinga0ν2βsourceanddetectingthisdecayinthesamematerialisveryattractivebecauseitallowsonetosignicantlyincreasethedetectionefciency.
Themainrequirementsforapotentialscintillatorareahighspeciclightyieldwithshortdecaytimes(lessthan10–4s),theabsenceofradioactiveisotopesfortheotherelementsinthecrystalcomposition,lowdensity,andsmalleffectiveatomicnumberZ(whichisneces-sarytoreducebackground).
Untilnow,themostatten-tionhasbeenpaidtothescintillationpropertiesofmolybdateswithascheelite-typestructure:AMoO4(A=Ca,Cd,Sr,Ba,Pb).
However,allofthecrystalsstudiedhaveanumberofdrawbacks,i.
e.
,lowlumines-cenceintensity,evenatlowtemperatures(BaMoO4);largeZ(PbMoO4);orthepresenceofradioactivecationisotopes(CdMoO4,PbMoO4).
Currently,CMoO4crystalsareconsideredasthemostappropriate.
How-ever,the48Caisotopeisasourceofthe2ν2βdecay,whichformsanunremovablebackgroundindetection.
Themagnesiummolybdate(MgMoO4)canbeanalter-nativetocrystalswithscheelitestructures.
Theabsenceoflong-livedradioactiveisotopesofmagnesium,aswellasthelowdensityofMgMoO4(4.
04g/cm3)areadvantagesincomparisonwithscheelitecrystals.
MgMoO4luminescencewasobservedforthefirsttimein[5];however,amoredetailedinvestigationoftheluminescenceandopticalpropertiesofthiscompoundhavebeenperformedonlyrecently[6–8].
Inthispaper,wereporttheresultsofstudyingtheluminescencecharacteristicsofMgMoO4crystalsgrownunderdifferentconditions.
Thereectionspec-traofMgMoO4weremeasuredforthersttimetakingintoaccountthecrystalstructureanisotropy.
Recently,muchattentionhasbeenfocusedoncharge-transferluminescence,whichwasobservedinanumberofoxy-gen-containingcompoundsdopedwithYb3+ions[9].
Therefore,wehavestudiedtheluminescencepropertiesofMgMoO4:Ybcrystals.
EXPERIMENTALTheluminescenceVUV-excitationspectra,trans-missionspectranearthefundamentalabsorptionedge,andluminescenceexcitationandreectionspectraintheenergyrange3.
7–25eVweremeasuredontheSuperlumisystem(DEZY,Hamburg)[10]attempera-turesof8–70Kandat300K.
TheluminescencespectraweremeasuredusinganARCSpectraProSP-308monochromator(inthespectrographmode)andnor-malizedtothespectralsensitivityfunctionofthedetectingcomplex.
Plane-parallelsurfacesoffreshcleavageswereanalyzed.
ThecrystalsunderstudyweregrownbytheCzo-chralskimethodinplatinumcruciblesinair.
SampleMgMoO4no.
1wasgrownfromastoichiometricmeltofMgOandMoO3ofhigh-puritygrade.
Thechargecom-ponentsweredriedandmixedinastoichiometricpro-portion,thenheatedinaplatinumcrucibleat700or930°Cfor6h.
X-raydiffractionanalysisshowedthatCONDENSED-MATTERSPECTROSCOPYLuminescencePeculiaritiesandOpticalPropertiesofMgMoO4andMgMoO4:YbCrystalsD.
A.
Spasskiia,V.
N.
Kolobanovb,V.
V.
Mikhalinb,L.
Yu.
Berezovskayac,L.
I.
Ivlevac,andI.
S.
VoroninacaSkobeltsynInstituteofNuclearPhysics,MoscowStateUniversity,Moscow,119992RussiabFacultyofPhysics,MoscowStateUniversity,Moscow,119991RussiacProkhorovGeneralPhysicsInstitute,RussianAcademyofSciences,Moscow,119991Russiae-mail:deris2002@mail.
ruReceivedOctober20,2008Abstract—Magnesiummolybdateisconsideredasapromisingmaterialforcryogenicscintillationbolometers.
TheluminescencepropertiesofMgMoO4havebeeninvestigatedonsinglecrystalsgrownfrommeltsofsto-ichiometricandnonstoichiometriccompositionsandoncrystalsdopedwithYb3+ions.
TheiropticalpropertiesareinterpretedtakingintoaccounttheanisotropyoftheMgMoO4crystalstructure.
PACSnumbers:78.
55.
-m,78.
40.
-qDOI:10.
1134/S0030400X09040171OPTICSANDSPECTROSCOPYVol.
106No.
42009LUMINESCENCEPECULIARITIESANDOPTICALPROPERTIES557synthesisat700°CleadstotheformationofbothMgMoO4andMg2Mo3O11phases.
At930°C,onlytheβ-MgMoO4phaseissynthesized.
Themeltingtemper-atureofMgMoO4is1320°C.
Thepullingraterangedfrom1to3mm/h.
SampleMgMoO4no.
2wasgrownfromanonstoichiometricmeltwithadecitofMoO3(3wt%).
SampleMgMoO4no.
3wasobtainedfromastoichiometricmeltwithadditionof5wt%Yb2O3.
β-MgMoO4isabiaxialanisotropiccrystalwiththeunit-cellparametersa=10.
273,b=9.
288,c=7.
025,andβ=106.
96°;itbelongstothespacegroupC2/m[11].
ItisknownthatthereflectionofanisotropicmolybdatecrystalsdependsontherelativeorientationoftheircrystallographicaxesandtheelectricvectorEoftheincidentpolarizedsynchrotronradiation.
There-fore,thesamplesunderstudywereorientedalongthecrystallographicaxesbyelectronbackscattereddiffrac-tion(EBSD)usingaCRYSTALattachment(OxfordInstrumentsINCASystem)toaJSM5910-10LV(Jeol)scanningelectronmicroscope.
ItwasshownthattheMgMoO4cleavageplanebelongsto{9110}crystallo-graphicplanes.
Thus,thecrystallographicaxiscliesintheMgMoO4cleavageplane.
Duringmeasurements,thesampleswereorientedsoastomakethecaxisbeeitherparallel(c||E)orperpendicular(c⊥E)totheprojectionoftheincidentradiationvectorEontothesampleplane.
RESULTSANDDISCUSSIONAbsorptionSpectraofMgMoO4TheabsorptionspectraatT=10and300KareshowninFig.
1.
Atatemperatureof10K,theabsorp-tioncoefcientbeginstosignicantlyincreaseat3.
9eV.
Withanincreaseintemperatureto300K,theabsorptionedgeundergoesaredshiftandbecomesat-ter.
SuchbehaviorischaracteristicofthefundamentalabsorptionedgeofdielectricsandcanbedescribedbytheUrbachformula[12]whereσistheslope,Tistemperature,kBistheBoltz-mannconstant,andα0andE0arethecoordinatesoftheintersectionpointsoftheUrbachedgesatdifferenttem-peratures,extrapolatedtolargeabsorptioncoefcients.
ApproximationoftheabsorptionspectrabytheUrbachformulagavethefollowingvaluesofσandE0forMgMoO4:σ=0.
32andE0=4.
49eVatT=300Kandσ=0.
02andE0=4.
57eVatT=10K.
NotethatE0isclosetotheexcitonpeakpositionintheabsorptionspectrumandthattheslopeparameterσdependsontemperatureandcangiveinformationaboutthepossi-bilityofexcitonself-trappinginthesecompounds[12].
ThereectionspectraofMgMoO4samplesmea-suredinthefundamentalabsorptionrangewererecal-culatedintoabsorptionspectra,becausethelatteryieldmoreexactenergiesofinterbandelectronictransitions.
Wealsocalculatedtheenergydependencesoftheimag-inaryandrealpartsofthepermittivityandenergy-lossfunction.
TherecalculationwasperformedaccordingtotheKramers–KronigrelationsusingfastFouriertrans-form[13].
Thesecalculationsrequireinformationaboutthebehaviorofthereectioncoefcientintheentirefrequencyrange.
Theexperimentalreectionspectraweremeasuredatenergiesfrom4to25eV.
Thereec-αE()α0σE0E–kBT-–,exp=0.
0203.
8Intensity,arb.
unitsPhotonenergy,eV4.
04.
20.
041234110α,cm–1Fig.
1.
(1,2)AbsorptionspectraofMgMoO4no.
1atT=(1)300and(2)10KandtheluminescenceexcitationspectrameasuredatT=10Kforsamples(3)MgMoO4no.
1(Elum=2.
3eV)and(4)MgMoO4no.
2(Elum=2.
0eV).
558OPTICSANDSPECTROSCOPYVol.
106No.
42009SPASSKIIetal.
tioncoefcientRwasextrapolatedintheenergyrangeE>25eVaccordingtothelawR~E–4.
Inthelow-energyregion,weappliedtheself-consistentprocedureforextrapolatingthereectioncoefcient,whichusestherefractiveindexasaparameter.
ThecriterionforrecalculationcorrectnesswasthevalidityoftheKram-ers–Kronigrelationsforthecalculatedpermittivity,aswellastheconvergenceofthecalculatedfourtypesofthesumrule,obtainedfromtheimaginarypartofthepermittivityandtheenergy-lossfunction.
TheobtainedabsorptionandintrinsicluminescenceexcitationspectraareshowninFig.
2.
Dependingonthesampleorientation,uptoeightabsorptionpeaksareobservedintheenergyrange4–25eV;theirenergypositionsatT=10KarelistedinTable1.
TheenergiesoftheabsorptionpeaksexceedsthevalueE0givenbytheUrbachformula.
Indeed,theabsorptionspectrumlacksanypronouncedstructureintheenergyrange4.
5–4.
6eV,whichcouldbeassignedtotheformationofanexcitonatthefundamentalabsorptionedge.
Theabsenceofexcitonischaracteris-ticofmolybdateswithscheelitestructure,forexample,CaMoO4andSrMoO4,forwhichthelow-energyreflec-tionpeakisdeterminedbyinterbandtransitions[14,15].
However,onecannotexcludethattheexcitonpeakishiddenduetothepresenceofstructuraldefectsintheMgMoO4samplesunderstudy.
Forexample,for0.
506Intensity,arb.
unitsPhotonenergy,eV481510202512*6(b)0.
5*10601.
0*1060.
5012*6(a)0.
5*10601.
0*1061.
01.
5*106α,cm–1Fig.
2.
(1)AbsorptionspectracalculatedaccordingtotheKramers–Kronigrelationsfromtheexperimentalreectionspectraand(2)intrinsicluminescenceexcitationspectra(Elum=2.
3eV)foraMgMoO4crystalwiththeorientations(a)c||Eand(b)c⊥E.
Thearrowsindicatethemostpronouncedantibaticpeculiaritiesintheabsorptionandluminescenceexcitationspectra.
Table1.
AbsorptionpeakenergiesforMgMoO4atdifferentorientationsofthecrystallographicaxiscwithrespecttotheprojectionoftheelectricvectorEofsynchrotronradiationincidentonthesamplesurface(T=10K)CrystalorientationAbsorptionpeakenergies,eV123456789c||E5.
15.
86.
256.
99.
110.
614.
117.
0~21c⊥E5.
25.
75–6.
89.
111.
213.
916.
5~21OPTICSANDSPECTROSCOPYVol.
106No.
42009LUMINESCENCEPECULIARITIESANDOPTICALPROPERTIES559LuAlO3,reliableinformationabouttheexistenceofanexcitonpeakatthefundamentalabsorptionedgecouldbeobtainedonlybymeasuringthereectionfromthesurfaceofsingle-crystallmshavinglessstructuraldefectsincomparisonwithbulksinglecrystals[16].
Apparently,thelow-energyabsorptionpeakintheMgMoO4samplesunderstudyisduetotheinterbandelectronictransitions.
Thepositionoftherst-peakmaximumintheabsorptionspectrumyieldsthefollow-ingestimationfromabovefortheMgMoO4bandgap:Eg30K,theexperimentaltemperaturedepen-denceoftheluminescenceintensityforMgMoO4no.
1differsfromthatcalculatedfromtheMottformula.
Thereasonforthisisthatanadditionalbandarisesintheluminescencespectrumasaresultofthesignicantdecreaseintheintrinsicluminescenceintensity(approximatelybytwoordersofmagnitude).
Indeed,anincreaseintemperatureto30Kshiftsthebandpeakfrom2.
3to2.
0eV;thebandbecomesnonelementaryandcanberepresentedasasuperpositionoftwoele-mentaryluminescencebandspeakingat2.
3and1.
9eV.
Forsampleno.
2(grownfromachargewithadecitofMoO3),thenonelementaryluminescenceband,whichcanalsobedecomposedintoGaussiancomponents,isobservedevenatT=10K.
Theresultsofdecomposi-tionintoGaussiancomponentsforsamplesno.
1andno.
2arelistedinTable3.
Theexcitationspectrumoftheluminescencebandat1.
9eVnearthefundamentalabsorptionedge(Fig.
1)ischaracterizedbytheformationofanadditionalexci-tationbandat4.
0eV.
At10K,thisbandisinthetrans-parencyrangeofMgMoO4.
Therelativeweightofthelow-energyluminescencebandincreasesuponlumi-nescenceexcitationat4.
0eV.
Wecanconcludethattheluminescencebandat1.
9eVisduetothedefectscausedbythecrystaldeviationfromstoichiometry.
Twooverlappingluminescencebandsofcompara-bleintensitywereobservedfornominallypureMgMoO4atT=9K[6].
Thebandpeaksobtainedinthisstudyarered-shiftedby~0.
2eVwithrespecttothedataof[6].
Ourconclusionaboutthedefectnatureofthe1.
9-eVluminescencebandisinagreementwithoneofthesuggestionsmadein[6]aboutthenatureofthelow-energybandat~2.
1eV.
Notethattheabsenceofasignicantcontributionofthelow-energydefectbandatT=10KforsampleMgMoO4no.
1indicatesitshighstructuralquality.
Theluminescencespectrumofmagnesiummolyb-datedopedwithytterbiumions(sampleno.
3)contains,alongwiththeintrinsicluminescenceband,additionalnarrowluminescencebandsat1.
21,1.
24,and1.
27eV,whichcorrespondstothef–ftransitionsinYb3+ions[9].
Thecharge-transferluminescence,characteristicofsomecomplexoxides(garnets,perovskites,borates)dopedwithYb3+,wasnotobservedinMgMoO4:Yb.
Notethattheintrinsicluminescenceintensityforsamplesno.
2andno.
3ismuchlowerthanforsampleno.
1.
Apparently,thisisduetotheformationofcom-petingluminescencecenters,whicharerelatedtothepresenceofbothstructuraldefectsandimpuritycentersinthenonstoichiometric(no.
2)anddoped(no.
3)sam-ples.
Theluminescenceintensityofmagnesiummolyb-dateandsomeothermolybdatesandtungstatesatlowtemperatures(T=10K)wascomparedunderthesameexperimentalconditions.
Toexcludetheenergylossduetothethermalizationofchargecarriers,theexcita-tionenergyforeachcrystalwaschosentocorrespondtothefundamentalabsorptionedge.
Thisapproachmakesitpossibletoshowthepotentialspeciclightyieldofacrystalwhenthecompetingchannelsofnon-radiativeenergyrelaxationaresuppressed.
Compari-sonwasperformedforMgMoO4no.
1,ZnMoO4,BaWO4,andCdWO4samples.
MgMoO4,ZnMoO4,andBaWO4singlecrystalsweregrownbytheCzo-chralskimethodattheGeneralPhysicsInstitute,Rus-sianAcademyofSciences.
TheCdWO4samplewaspreparedfroma1-cm3crystalwithaspeciclightyieldTable3.
EnergiesandFWHMsoftheintrinsicluminescenceband(Emax1)andtheluminescencebandduetostructuralde-fects(Emax2)forMgMoO4no.
1andno.
2samplesatdifferenttemperaturesandexcitationenergiesCrystalT,KEex,eVEmax1,eVFWHM1,eVEmax2,eVFWHM2,eVMgMoO4no.
1105.
42.
310.
67––MgMoO4no.
1705.
42.
300.
691.
90(1.
21)0.
57MgMoO4no.
2105.
42.
310.
641.
92(0.
43)0.
53MgMoO4no.
2104.
02.
310.
651.
89(1.
28)0.
57Note:Theweightofalow-energyluminescencebandwithrespecttothehigh-energybandisgiveninparenthesesafterthemaximumvalue.
562OPTICSANDSPECTROSCOPYVol.
106No.
42009SPASSKIIetal.
of~20000photons/meVatT=300K;thecrystalwasgrownbytheCzochralskimethodattheScienticandTechnicalComplex"InstituteforSingleCrystals"(Kharkov,Ukraine).
ItisshownthattheluminescenceintensityofMgMoO4no.
1isanorderofmagnitudehigherthanthatofBaWO4butmuchlowerthantheluminescenceintensityofCdWO4andZnMoO4(byfactorsofabout40and10,respectively),whichcanbeaseriousproblemforpracticalapplicationofMgMoO4asascintillator.
LuminescenceExcitationSpectraTheintrinsicluminescenceexcitationspectraofMgMoO4no.
1areshowninFig.
2.
Thestructuralanisotropymanifestsitselfinthesespectra,whicharedifferentfordifferentorientationswithrespecttoE.
Apossiblereasonforthedifferenceintheexcitationspectraisthesurfacelossfactor.
Generally,theexcita-tionspectraaremodulatedbytheabsorptionspectra,duetowhichantibaticspecicfeaturesariseinthestructureoftheexcitationandabsorptionspectra[13].
Theantibaticspecicfeaturesthataremostpro-nouncedintheabsorptionandexcitationspectraareindicatedbyarrowsinFig.
2.
SincethepresenceandrelativeintensityoftheabsorptionpeaksinMgMoO4dependonthecrystalorientation,themodulationoftheexcitationspectraobtainedintheparallelandperpen-dicularorientationsofcandEwillbedifferent.
Notethatinthecaseofthec||Eorientationtheexci-tationspectrumcontainstwoadditionalpeaksat4.
05and4.
4eV.
Theyareabsentforthec⊥Eorientation,andtheirappearancecannotbeexplainedbythesurfacelossfactor,becausepronouncedabsorptionpeaksareabsentinthisenergyrange.
Atthesametime,theinten-sityoftheexcitationspectrumincreasessimultaneouslywiththeabsorptioncoefcient(Fig.
1,curves2,3).
Apparently,thepresenceofthesepeaksonlyatthesampleorientationc||Echaracterizestheanisotropyoftheenergybandlevelsnearthefundamentalabsorptionedge.
Thesignicant(byapproximatelyanorderofmag-nitude)decreaseintheluminescenceintensitywithanincreaseintheexcitationenergyfrom4.
5–5.
5to11–12eVindicatestheexcitontypeofenergytransfertointrinsicluminescencecentersinthepresenceofeffec-tivecompetingchannelofenergyrelaxation.
Anincreaseintheexcitationenergyincreasestheaveragedistancebetweenthefreeelectronandhole.
Intheabsenceofeffectivecompetingrelaxationchannel,theprobabilityforafreeelectronandholetoformanexci-tondependsweaklyontheexcitationenergyintherangefromthefundamentalabsorptionedgetothebeginningofphotonmultiplication.
Thisbehaviorwasobserved,forexample,fortheluminescenceexcitationspectrumofself-trappedexcitonsinundopedCdWO4[26].
Inthepresenceofacompetingrelaxationchannel,theprobabilityofexcitonformationdecreaseswithanincreaseintheaveragedistancebetweenanelectronandhole.
SinceonlyoneluminescencebandisobservedintheMgMoO4no.
1crystalatT=10K(Fig.
4,curve1),thecompetingchannelisnonradiative.
Atphotonenergiesabove12eVtheintensityofexcitationspectraincreasesduetotheformationoflow-energyelectron–holepairsasaresultofmultipli-cationofelectronicexcitations.
CONCLUSIONSTheluminescenceandopticalpropertiesofMgMoO4crystalsgrownfromstoichiometricandnon-stoichiometricmeltsandcrystalsdopedwithYb3+ionswereinvestigated.
Considerationofthestructuralanisotropymadeitpossibletoexplainthemainpecu-liaritiesoftheabsorptionspectraintheenergyrange4–25eV.
Thebandgapisestimatedtobeintherange4.
5
Itisshownthattheintrinsiclumines-cenceat2.
3eViscausedbytheemissionofexcitonsself-trappedatMoO4complexes.
Theexistenceofaneffectivenonradiativechannelofenergyrelaxation,whichcompeteswithintrinsicluminescencecenters,isestablished.
Itisconcludedthattheluminescencebandat1.
9eVisduetothedefectsofthesamplecrystalstructure.
DopingofMgMoO4withytterbiumionsleadstotheoccurrenceofimpurityluminescencebandsintheIRregion;charge-transferluminescenceisnotobservedinthiscase.
Theintrinsicluminescenceinten-sityofMgMoO4at10KismuchlowerthanthatofCdWO4andZnMoO4;hence,applicationofthiscrystalasancryogenicscintillatorislimited.
ACKNOWLEDGMENTSThisstudywassupportedbygrantDFG436RUS113/437/0-3andtheRussianFoundationforBasicResearch,projectno.
06-02-16339.
WearegratefultoG.
ZimmererforpermittingexperimentsonSUPER-LUMIandtoG.
Striganyukforhishelpinmeasure-ments.
WearealsogratefultoD.
ShaturaforsupplyingtheX-raydiffractiondata,L.
Iskhakovaforthesampleorientation,andI.
TupitsinaandL.
Nagornayaforsup-plyingtheCdWO4sample.
REFERENCES1.
M.
Minowa,K.
Itakura,S.
Motiyama,andW.
Ootani,Nucl.
Instrum.
MethodsPhys.
Res.
,Sect.
A320,500(1992).
2.
A.
N.
Annenkov,O.
A.
Buzanov,F.
A.
Danevich,etal.
,Nucl.
Instrum.
MethodsPhys.
Res.
,Sect.
A584,334(2008).
3.
S.
Belogurov,V.
Kornoukhov,A.
Annenkov,etal.
,IEEETrans.
Nucl.
Sci.
52(4),1131(2005).
4.
S.
Pirro,S.
Capelli,M.
Pavan,etal.
,http://arxiv.
org/abs/nucl-ex/0510074v1.
OPTICSANDSPECTROSCOPYVol.
106No.
42009LUMINESCENCEPECULIARITIESANDOPTICALPROPERTIES5635.
M.
Amberg,J.
R.
Gijnter,H.
Schmalle,andG.
Blasse,J.
SolidStateChem.
77,162(1988).
6.
V.
B.
MikhailikandH.
Kraus,J.
Phys.
D:Appl.
Phys.
39,1181(2006).
7.
V.
B.
Mikhailik,H.
Kraus,M.
Itoh,etal.
,J.
Phys.
:Con-dens.
Matter17(46),7209(2005).
8.
I.
V.
Kitaeva,V.
N.
Kolobanov,V.
V.
Mikhailin,etal.
,inProc.
8thInt.
Conf.
onInorganicScintillatorsandTheirUseinScienticandIndustrialApplications,Alushta,Ukraine,2005,p.
44.
9.
I.
Kamenskikh,N.
Guerassimova,A.
Dujardin,etal.
,Opt.
Mater.
24,267(2003).
10.
G.
Zimmerer,Radiat.
Meas.
42(4–5),859(2007).
11.
V.
V.
Babakin,R.
F.
Klevtsova,andL.
A.
Gaponenko,Kristallograya27(1),38(1982)[Sov.
Phys.
Crystal-logr.
27,20(1982)].
12.
K.
S.
SongandR.
T.
Williams,Self-TrappedExcitons,2nded.
,SpringerSeriesinSolid-StateSciences(Springer,Berlin,1996),Vol.
105.
13.
V.
V.
MikhalinandA.
N.
Vasil'ev,IntroductiontoSolid-StateSpectroscopy(Izd-voMGU,Moscow,1987)[inRussian].
14.
R.
Grasser,E.
Pitt,G.
Zimmerer,etal.
,Phys.
StatusSolidiB69,359(1975).
15.
D.
Spassky,S.
Ivanov,I.
Kitaeva,etal.
,Phys.
StatusSolidiC2(1),65(2005).
16.
V.
Kolobanov,V.
Mikhailin,N.
Petrovnin,D.
Spassky,andYu.
Zorenko,Phys.
StatusSolidiB243(8),R60(2006).
17.
J.
A.
Rodriguez,J.
C.
Hanson,S.
Chaturvedi,etal.
,J.
Chem.
Phys.
112(2),935(2000).
18.
W.
Lotz,J.
Opt.
Soc.
Am.
60(2),206(1970).
19.
V.
N.
Kolobanov,I.
A.
Kamenskikh,V.
V.
Mikhailin,etal.
,Nucl.
Instrum.
MethodsPhys.
Res.
,Sect.
A486(1–2),496(2002).
20.
E.
G.
Reut,Izv.
Akad.
NaukSSSR,Ser.
Fiz.
49(10),2032(1985).
21.
Y.
Zhang,N.
A.
W.
Holzwarth,andR.
T.
Williams,Phys.
Rev.
B57(20),12738(1998).
22.
W.
VanLoo,Phys.
StatusSolidiA27,565(1975).
23.
N.
F.
Mott,Proc.
R.
Soc.
London,Ser.
A167,384(1938).
24.
N.
Saito,N.
Sonoyama,andT.
Sakata,Bull.
Chem.
Soc.
Jpn.
69,2191(1996).
25.
D.
Spassky,V.
Kolobanov,V.
Mikhailin,etal.
,Phys.
Sta-tusSolidi(inpress).
26.
O.
V.
Rzhevskaya,D.
A.
Spasski,V.
N.
Kolobanov,etal.
,Opt.
Spektrosk.
104(3),407(2008)[Opt.
Spec-trosc.
104,366(2008)].
TranslatedbyYu.
Sin'kov
- dependsjavmoo.com相关文档
- completejavmoo.com
- commonalityjavmoo.com
- Settingjavmoo.com
- "序号","课程名称","课程课程负责人","联系电话","课程团队","开课平台","课程网址","班级","备注"
- studyjavmoo.com
- Facebookjavmoo.com
knownhost西雅图/亚特兰大/阿姆斯特丹$5/月,2个IP1G内存/1核/20gSSD/1T流量
美国知名管理型主机公司,2006年运作至今,虚拟主机、VPS、云服务器、独立服务器等业务全部采用“managed”,也就是人工参与度高,很多事情都可以人工帮你处理,不过一直以来价格也贵。也不知道knownhost什么时候开始运作无管理型业务的,估计是为了扩展市场吧,反正是出来较长时间了。闲来无事,那就给大家介绍下“unmanaged VPS”,也就是无管理型VPS,低至5美元/月,基于KVM虚拟,...
HostKvm($4.25/月),俄罗斯CN2带宽大升级,俄罗斯/香港高防限量5折优惠进行中
HostKvm是一家成立于2013年的国外VPS服务商,产品基于KVM架构,数据中心包括日本、新加坡、韩国、美国、俄罗斯、中国香港等多个地区机房,均为国内直连或优化线路,延迟较低,适合建站或者远程办公等。本月,商家旗下俄罗斯、新加坡、美国、香港等节点带宽进行了大幅度升级,俄罗斯机房国内电信/联通直连,CN2线路,150Mbps(原来30Mbps)带宽起,目前俄罗斯和香港高防节点5折骨折码继续优惠中...
CloudCone(20美元/年)大硬盘VPS云服务器,KVM虚拟架构,1核心1G内存1Gbps带宽
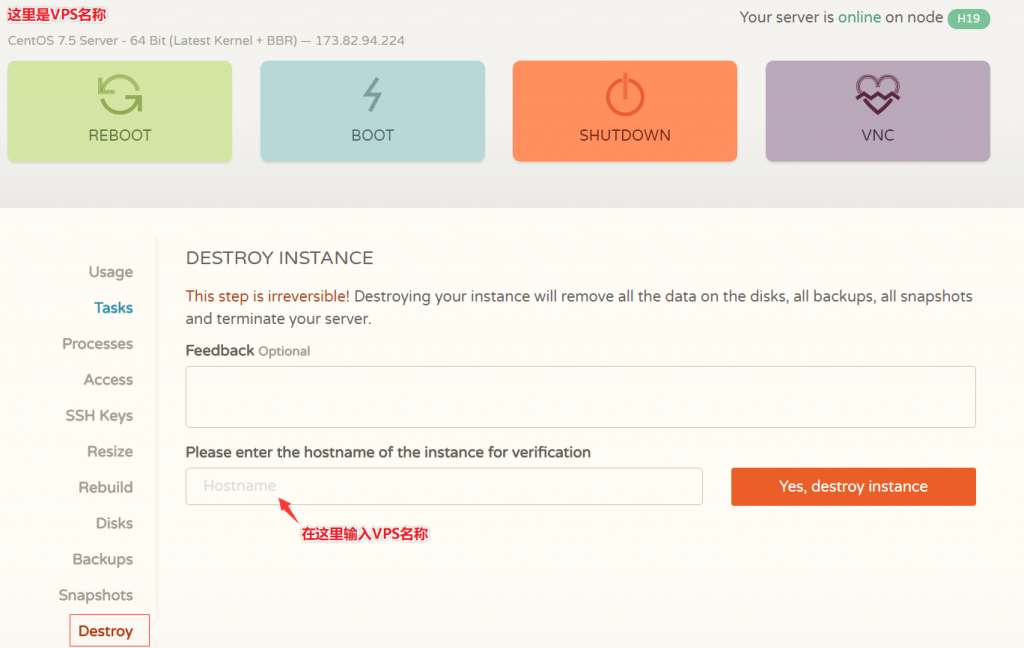
近日CloudCone商家对旗下的大硬盘VPS云服务器进行了少量库存补货,也是悄悄推送了一批便宜VPS云服务器产品,此前较受欢迎的特价20美元/年、1核心1G内存1Gbps带宽的VPS云服务器也有少量库存,有需要美国便宜大硬盘VPS云服务器的朋友可以关注一下。CloudCone怎么样?CloudCone服务器好不好?CloudCone值不值得购买?CloudCone是一家成立于2017年的美国服务...
javmoo.com为你推荐
-
12306崩溃iphone 12306网络错误sonicchat深圳哪里有卖汽车模型?Baby被问婚变绯闻小s在黄晓明婚礼上问了什么问题百度商城百度商城知道在哪个地方,怎么找不到啊22zizi.com河南福利彩票22选52010175开奖结果月神谭适合12岁男孩的网名,要非主流的,帮吗找找,谢啦www.niuav.com在那能找到免费高清电影网站呢 ?4400av.com在www.dadady.com 达达电影看片子很快的啊partnersonline国外外贸平台有哪些?梦遗姐我姐姐很漂亮,她24了,我才15,晚上我和他睡在一起,我经常挨遗精,咋办?