atmosphereios11
ios11.0.2 时间:2021-02-02 阅读:()
ContentslistsavailableatScienceDirectNanoEnergyjournalhomepage:www.
elsevier.
com/locate/nanoenFullpaperIndacenodithiophene-basedwidebandgapcopolymersforhighperformancesingle-junctionandtandempolymersolarcellsYunlongMaa,b,Shan-CiChena,ZaiyuWangc,WeiMac,JinyunWanga,ZhigangYina,b,ChangquanTanga,b,DongdongCaia,QingdongZhenga,aStateKeyLaboratoryofStructuralChemistry,FujianInstituteofResearchontheStructureofMatter,ChineseAcademyofSciences,155YangqiaoWestRoad,Fuzhou,Fujian350002,ChinabUniversityofChineseAcademyofSciences,19YuquanRoad,Beijing100049,ChinacStateKeyLaboratoryforMechanicalBehaviorofMaterials,Xi'anJiaotongUniversity,Xi'an710049,ChinaARTICLEINFOKeywords:IndacenodithiopheneTandemdevicesElectrontransportlayerFibrousnetworkPolymersolarcellsABSTRACTTwowidebandgapcopolymersbasedonbulkyindacenodithiophene(IDT)andalkoxylatedbenzothiadiazoleunits(PIDTBTO-TandPIDTBTO-TT)withthethiopheneorthieno[3,2-b]thiophene(TT)π-bridgearedesignedandsynthesized.
Theeectofπ-bridgeontheπ-πpacking,optical,carriertransport,nano-sizedphaseseparationandphotovoltaicpropertiesofthecopolymersareinvestigatedindepth.
IncomparisonwiththePIDTBTO-T-basedcounterpart,thebestperformancesolarcellbasedonPIDTBTO-TTexhibitsahigherpowerconversioneciency(PCE)of8.
15%whichismainlyattributedtotheformationofabrousnetworkfortheactivelayerbasedonPIDTBTO-TT.
Furthermore,whenanovelhybridelectrontransportlayer(PDIN:PFN)isintroducedintoatandemsolarcellusingthePIDTBTO-TT-baseddeviceandaPTB7-Th-baseddeviceasthebottomandtopcellcomponents,respectively,theresultingsolarcellexhibitsanoutstandingPCEof11.
15%withalargeopencircuitvoltageof1.
70V.
Tothebestofourknowledge,thePCEsof8.
15%and11.
15%arethehighestvaluesreportedtodateforthesingle-junctionandtandemsolarcellsusingIDT-basedcopolymers,respectively.
Ourresultsdemonstratethattheπ-bridgemodulationiseectiveinadjustingthechargecarriermobilityandphotovoltaicperformanceofIDT-basedwidebandgapcopolymersforsingle-junctionandtandemdevices.
1.
IntroductionPolymersolarcells(PSCs)usingp-typeconjugatedpolymersandn-typefullerenederivativeshaveattractedincreasingattentionduetotheiradvantagesoflowcost,lightweight,andgoodexibility[1].
Inthepastdecade,extensiveeortshavebeendevotedtodevelopingnewp-typelowbandgapconjugatedpolymersforthephotovoltaicapplication.
Asaresult,signicantprogresseshavebeenmadeinthiseld,andpowerconversioneciencies(PCEs)over10%havebeenachievedforbothsingle-junctionandtandemPSCs[2].
Forsingle-junctionPSCsbasedonlowbandgapcopolymers,itisachallengetosimultaneouslyimprovetheshortcircuitcurrentdensity(JSC)andopencircuitvoltage(VOC)valuesbecausethereisatrade-obetweenhighcurrentandlargevoltagevalues.
Asaresult,amulti-junctiondevicewithatandemstructureisdesignedasanalternativetobroadenthelight-harvestingspectraandtoincreasetheVOCvaluesofPSCs.
Foratandemdevice,twocellswithbulk-heterojunction(BHJ)layershavingcomplementaryabsorptionspectraareusuallyneeded,whereabottomcellutilizesawidebandgapmaterialandatopcellusesalowbandgapmaterial.
AlthoughthemaximumPCEupto15%couldbeexpectedaccordingtomodelcalculations[3],bynow,thereareonlyacoupleexamplesofdouble-junctiontandemPSCswithecienciesover11%[3b,c,h],whichismainlyattributedtothelackofsuitablepairofabsorbingmaterialswithdierentbandgapsandcomplementaryabsorptionspectra.
Specically,manyhighperformancelowbandgapcopolymershavebeenreportedinthepastdecade[2].
However,relativelyfewerhighperformancewidebandgapcopolymersareavailablefortandemdevices[4],andmostofpolymertandemdeviceshavetousepoly(3-hexylthiophene)(P3HT,1.
90eVbandgap,HOMO=5.
10eV)astheshort-wavelengthabsorbingmaterial,whichexhibitsarelativelylowVOC(whenblendedwithPC71BM)[2f,3d–f].
Anotherchallengeforpolymertandemdevicesisrelatedtothefactthatthereareonlyfewinterfaciallayersavailabletoconnectdierentsubcellseciently[2e,5].
Therefore,bothecientsolution-processedinterfaciallayersandnewwidebandgapmaterialsareneededforhigheciencytandemsolarcells.
http://dx.
doi.
org/10.
1016/j.
nanoen.
2017.
01.
050Received15December2016;Receivedinrevisedform17January2017;Accepted24January2017Correspondingauthor.
E-mailaddress:qingdongzheng@fjirsm.
ac.
cn(Q.
Zheng).
NanoEnergy33(2017)313–324Availableonline25January20172211-2855/2017ElsevierLtd.
Allrightsreserved.
MARKIndesigninghighperformancewidebandgappolymers,awidelyadoptedstrategyistoalternatesuitableelectron-rich(donor,D)andelectron-decient(acceptor,A)buildingblocksinthepolymerback-bone.
Asbothdonorandacceptorunitsareconsistedofplanararomaticringswithlittleconformationexibility,theconnectionbetweendonorandacceptorunitsbecomesveryimportantindeter-miningthebackboneplanaritywhichsubsequentlyaectstheenergylevels,bandgapsandchargetransportpropertiesoftheresultingD-Acopolymers.
Inthepast10years,in-depthstudiesontheconnectionortheπ-bridgebetweendonorandacceptorunitsaremoreorlessneglectedinspiteofmanydonororacceptorunitsdeveloped[1,6].
Eectiveπ-bridgemodulationstrategiesshouldconsiderthechemicalstructuredierenceofthegivenalternatingdonorandacceptorunits.
Forthecopolymersoriginatedfrombothve-membered-ring-baseddonorandacceptorunits,usuallynoπ-bridgeisneeded.
However,forcopolymersfromasix-memberedring-basedunit(suchasbenzothia-diazole,BT),asuitableve-memberedring-basedπ-bridgeisrequiredanditwillplayanimportantroleindeterminingtheperformanceoftheresultingcopolymers.
π-Bridgessuchasthiophene,bithiophene,thieno[3,2-b]thiophene(TT),andotherve-membered-ring-basedaromaticunitscanbeusedforcopolymers[1g,4a,6,7].
Amongtheseπ-bridges,TTstandsoutasanexcellentcandidateduetoitslinearandextendedbackbonestructurewhichmayreducethetorsionalanglebetweentwoplanaralternatingunitswhilenotgreatlydisturbingtheelectrontransferbetweendonorandacceptorunits.
Withtheafore-mentionedconsideration,theincorporationofTTasaπ-bridgeintocopolymerswithsomeknowndonorandacceptorunitsmayleadtopolymerswithunexpectedperformance,especiallyinthecaseofbulkyunit-basedcopolymers.
5,6-Bis(alkoxy)benzo[1,2,5]thiadiazole(BTO)isatypicalacceptorunitforwidebandgapcopolymers[8a].
Wepreviouslyexploredseveralseriesofcopolymersusingdierentlad-der-typearomaticunitsasdonorsandBTOunitsasacceptors[4e,8b,c].
Amongthem,ahighPCEof7.
26%withaVocof0.
94Vwasachievedfromthealternatingcopolymerbasedondiindenocarbazoleand5,6-bis(butoxy)benzo[c][1,2,5]thiadiazoleunits[8b].
Indacenodithiophene(IDT)isaladder-typedonorunitfeaturingacoplanarandextendedladder-typeframeworkthatcanelongateeectiveπ-conjugationlengthandfacilitatethechargecarriermobility.
SinceKoetal.
developedtherstIDT-basedcopolymerwithaPCEof4.
4%[9],alargenumberofIDT-containingcopolymershavebeensynthesizedbythecopolymerizationbetweenvariousacceptorunitsanddierentIDTderivatives[10].
OnethingdeservestobementionedisthattheIDTcanbealsousedfortheconstructionofn-typesemiconductingmaterialsasecientnon-fullereneacceptors[11].
ConsideringthefactthatbothBTOandIDTarebulkyaromaticunitswithlongalkylchains,asuitableπ-bridgemodulationmaygreatlyaecttheperformanceofthecopolymersbasedonthem.
Itisexpectedthatcopolymerswithamorelinearbackbonestructureandalargerspacebetweenthelongalkylchainsmayshowbetterphotovoltaicperformance.
Moreimportantly,theintroductionofπ-bridgeintotheIDT-BTOpolymerbackbonemayleadtoD-Acopolymersexhibitingwidebandgaps[4,7b].
Inthiscontext,wereporttwonovelDAcopolymers(PIDTBTO-T,PIDTBTO-TT)basedonanIDTdonorunitandaBTOacceptorunitspacedwiththiopheneandTTπ-bridges,respectively(Scheme1).
Theeectsoftheπ-bridgeonthepolymericcurvature,π-πpacking,optical,electrochemical,nano-sizedphaseseparation,carriertransportandphotovoltaicpropertiesofthetargetcopolymersareinvestigatedindetail.
ConventionalPSCsbasedonthetwocopolymersasthedonorsand[6,6]-phenylC71-butyricacidmethylester(PC71BM)astheacceptorhavebeenfabricated.
Asexpected,thecopolymerwithTTspacers(PIDTBTO-TT)showsasuperiorPCEupto7.
08%,whilePIDTBTO-TexhibitsamoderatePCEof6.
40%.
Afteraddingasmallamountof1,8-diiodooctane(DIO)asaprocessingadditive,thePIDTBTO-TT-baseddeviceexhibitsanincreasedPCEof8.
15%.
Forthersttime,ahybridelectrontransportlayerbasedonpoly[(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-uorene)-alt-2,7-(9,9-dioctyl-uorene)](PFN,Fig.
S1,SI)[2e]and2,9-bis(3-(dimethylamino)propyl)anthrax[2,1,9-def:6,5,10-d′e′f′]diisoquinoline-1,3,8,10(2H,9H)tetraone(PDIN,Fig.
S1,SI)[5a]isusedtofabricateatandemPSCwithPIDTBTO-TT:PC71BMasthebottomcellcomponent.
Thebestperfor-mancetandemdeviceexhibitsahighPCEof11.
15%withalargeVocof1.
70V,aJscof9.
68mAcm2,andadecentFFof67.
79%.
TheobtainedPCEsof8.
15%and11.
15%arethehighestvaluesreportedtodateforsingle-junctionandtandemcellsusingIDT-basedwidebandgapcopolymers,respectively.
2.
Resultsanddiscussion2.
1.
PolymersynthesisandcharacterizationThecopolymersPIDTBTO-TandPIDTBTO-TTwerepreparedviaStillecross-couplingpolymerizationbetweenthecorrespondingmono-mers(Scheme1)intolueneusingPd2(dba)3/P(o-tolyl)3orPd(PPh3)4catalyticsystem.
DuetotherelativelyhigherreactivityoftheBTO-TTmonomerincomparisonwiththeBTO-Tmonomer(Scheme1),alessreactivecatalyst,i.
e.
Pd(PPh3)4,wasusedforthesynthesisofPIDTBTO-TT,andamorereactivecatalyst,i.
e.
Pd2(dba)3/P(o-tolyl)3,wasusedforthesynthesisofPIDTBTO-T.
Furthermore,dierentcopolymerizationtimeswereusedtocontrolthemolecularweightsandtoensuregoodsolubilityofthetargetcopolymers.
Thenalcopolymersforthedevicefabricationwerefromthebatcheswiththehighestmolecularweights,andwithgoodsolubilityinchloroform,chlorobenzene,ando-dichlorobenzene(DCB).
Beforequenchingthepolymerizations,2-tributylstannylthiopheneand2-bromothiophenewereaddedsequentiallytoend-capthepolymerchainswiththiophene.
Thispreventsthereactiveendgroupsfromactingaschargetrappingsitesindevices.
Thecrudecopolymerswereprecipitatedinmethanol,andpuriedbySoxhletextractionwithmethanol,acetone,hexaneanddichloromethaneinsequencetoremoveanyresidualcatalystandlowmolecularweightoligomers.
Thenumberaveragemolecularweights(Mns)andpolydispersityindexes(PDIs)ofpolymersweremeasuredbygelpermeationchro-matography(GPC)using1,2,4-trichlorobenzeneastheeluentat150°C.
TheMnsofPIDTBTO-TandPIDTBTO-TTare97.
5and119.
9kDa,withPDIsof2.
5and1.
8,respectively(Table1).
Withsixlongalkylchainsoneveryrepeatunit,thesehighmolecularweightcopolymersstillhavegoodsolubilityincommonsolventssuchaschloroform,chlorobenzeneetc.
Thehighmolecularweightisdesirablebecauseitwillhelptoimprovethelm-formingabilityandtheresultingphotovoltaicperformance.
Thethermalstabilityofthecopolymerswasinvestigatedbythermogravimetricanalysis(TGA)(Fig.
S2,SI).
AsshowninFig.
S2,thetwocopolymershavegoodthermalstabilitywithdecompositiontemperatures(5%weightloss)over300°Cunderanitrogenatmosphere.
X-raydiraction(XRD)(Fig.
S3,SI)doesnotshowcleardiractionpeaksintherangeof2θ=0–20°,whichindicatesthatbothtwocopolymershavesimilaramorphouscharacteristicsasotherIDT-basedpolymers[9,10].
2.
2.
OpticalandelectrochemicalpropertiesTheUV–VisabsorptionspectraofPIDTBTO-TandPIDTBTO-TTinchloroformsolution(1*105M)andthinlmareshowninFig.
1andthedetailedopticalparametersaresummarizedinTable1.
Inbothsolutionandthinlm,PIDTBTO-TandPIDTBTO-TTexhibitverysimilarabsorptioncharacteristicsduetotheirapproximatepolymerbackbones.
Thehigherandlowerenergybands(300–500and500–700nm,respectively)areattributedtolocalizedπ-π*transitionsandintramolecularD-Achargetransfer,respectively.
PIDTBTO-TTexhibitsred-shiftedabsorptioninsolutioncomparedtoPIDTBTO-T,withtheabsorptionpeakgoingfrom574to585nm.
ThiscouldbeexplainedbythefactthatthehigherlinearityandextendedlengthoftheTTπ-bridgeY.
Maetal.
NanoEnergy33(2017)313–324314mayincreasetheplanarityofthepolymerbackboneandextendtheeectiveπ-conjugationlengthwhichissupportedbytheoreticalstudiesinthecomingsection.
Ingoingfromsolutiontosolidlm(Fig.
1b),negligiblechangesareobservedfortheabsorptionproles,exceptthatthereare18nmred-shiftedabsorptionpeaksforthethinlms.
Thisphenomenoncanbeassignedtothehigherstructuralorganizationandmoreorderedpackinginducedbythestrongerintermolecularinterac-tioninthesolidstate.
Theopticalbandgaps(Egopt)deducedfromtheabsorptionedges(λedge)ofbothpolymerlmsarearound1.
87eV.
Inordertodeterminetheenergylevelsandelectrochemicalproper-tiesofthecopolymers,thecyclicvoltammetry(CV)experimentsofthecopolymersinthinlmswereconducted.
ThevoltammogramsareshowninFig.
1candthedetailedresultsaresummarizedinTable1.
Theonsetpositionsofthep-dopingprocessofPIDTBTO-TandPIDTBTO-TTareat0.
57Vand0.
59VversusAg/Ag+,correspondingtothehighestoccupiedmolecularorbital(HOMO)levels(i.
e.
,ioniza-tionpotentials)of5.
39and5.
41eV,respectively.
Thelowestunoccupiedmolecularorbital(LUMO)levels(i.
e.
,electronanities)forPIDTBTO-TandPIDTBTO-TTare3.
48and3.
51eV,respec-tively,accordingtotheironsetreductionpotentials.
ThetrendofthesemeasuredenergylevelscoincideswellwiththecalculatedresultsbytheDFTcalculation(Fig.
S4,SI).
TherelativelydeepHOMOenergylevelScheme1.
SynthesisoftargetIDT-basedwidebandgapcopolymers.
Table1Summaryofthemolecularweights,optical,andelectrochemicalpropertiesofthecopolymers.
PolymerMnPDIλmax(nm)λmax(nm)EgoptHOMOLUMO(kDa)inCHCl3infilm(eV)a(eV)b(eV)cPIDTBTO-T97.
52.
55745931.
875.
393.
48PIDTBTO-TT119.
91.
85856021.
875.
413.
51aEstimatedfromtheonsetoftheabsorptionspectraofthinlms.
bEHOMO=(φox+4.
82)eV.
cELUMO=(φred+4.
82)(eV).
Fig.
1.
UV–visabsorptionspectraina)solutionandb)thinlm;c)cyclicvoltammogramsandd)energyleveldiagramofthecopolymers.
Y.
Maetal.
NanoEnergy33(2017)313–324315below5.
39eVofacopolymerisadvantageoustoobtainPSCswithhighVocs.
2.
3.
ConformationalanalysisbycalculationTogainmoreinsightintotheelectronicstructuresofthepolymerand,inparticulartheeectoftheπ-bridgeonthemolecularstructuresandelectronicproperties,computationalstudieswereperformedbydensityfunctionaltheory(DFT,B3LYP/6–311G**level)withachainlengthofn=2.
Hexylandoctylgroupsarereplacedbymethylgroupstosimplifythecalculation.
Forbothdimers,theHOMOwavefunctionsaredelocalizedalongthepolymerbackbone,whiletheLUMOwavefunctionsaremorelocalizedattheacceptorunits(Fig.
2).
Meanwhile,amoreextendeddelocalizationoftheHOMOwasobservedforPIDTBTO-TTcomparedtothatforPIDTBTO-T.
AsshowninFig.
2,thesetwocopolymerbackbonesshowsimilarlinearconformationintheirtopviews.
However,sideviewsshowthatPIDTBTO-Thasafairlycurvedbackbonewithatorsionalangleof~19°(TableS1,SI)betweenthetwoclosestIDTunitsalongthebackbone.
Onthecontrary,PIDTBTO-TTshowsahigherplanaritywithaverysmalltorsionalangleof~1.
6°,whichwouldfacilitateπ-electrondelocalizationandenhancechargecarriermobility.
Theseresultsimplythatthereplace-mentofthethiopheneπ-bridgewiththeTTunithasalargeeectonthegeometryofthepolymerbackbones.
2.
4.
FieldeecttransistorsToevaluatetheeectofstructuralmodicationofpolymersontheirholemobility,organiceld-eecttransistors(OFETs)basedonpristinePIDTBTO-TandPIDTBTO-TT,andwithabottom-gatetop-contactdevicecongurationwerefabricatedonahighlyn-dopedsiliconwafer.
TypicaloutputandtransfercurvesareshowninFig.
3,andtheparametersaresummarizedinTable2.
Ascanbeseenfromtheirtransfercurves,bothpolymersexhibittypicalp-typecharacter-istics.
ThecalculatedholemobilitiesforPIDTBTO-TandPIDTBTO-TTare(3.
3±0.
3)*103and(9.
1±0.
1)*103cm2V1s1,respectively.
Thedevicesbasedonbothtwocopolymersexhibitclearon/obehaviorwhichwillminimizeleakagecurrents.
TheholemobilityofPIDTBTO-TTismorethantwotimeshigherthanthatofPIDTBTO-Twhichisassociatedwiththegreaterbackboneplanarityfortheformer.
TheresultsconrmthattheinsertionoftheTTπ-bridgecanimprovethechargetransportpropertyofIDT-basedcopolymerswhichmayberelatedtotheimprovedπ-πstackinginthinlm.
Obviously,comparedtoPIDTBTO-T,PIDTBTO-TThassomeadvantagesinphotovoltaicapplicationswithitshigherholemobility,althoughtheseOFETmobilitiesonlyrepresentthechargetransportpropertyofpurepolymerlminthelateraldirectionofsubstrate.
Grazingincidentwide-angleX-ray(GIWAXS)[12]isfurtheremployedtoinvestigatethemolecularpackingandcrystallinityofpurePIDTBTO-TandPIDTBTO-TTlms.
The2DGIWAXSpatternsofbothpolymersareshowninFig.
4a,bandcorresponding1Dline-cutsinthein-planeandout-of-planedirectionsareshowninFig.
4c.
BothPIDTBTO-TandPIDTBTO-TT-basedlmsshowgentlelamellar(100)peaksinthein-planeandout-of-planedirectionsandnomoreotherlamellar(h00)peaksareobserved.
Thisindicatesthatbothpolymershaveweaklamellarmolecularpacking,whichcorrespondstotheamorphousnatureofthesecopolymers.
Thestrongout-of-plane(010)π-πstackingpeakslocatedat1.
441and1.
481(d-spacingsof4.
36and4.
25)forPIDTBTO-TandPIDTBTO-TT,respectively,suggestthatbothpolymershaveapreferentialface-onorientation.
The(010)peakintensityofPIDTBTO-TTishigherthanthatofPIDTBTO-T,andPIDTBTO-TThasacloserπ-πpackingdistancethanPIDTBTO-T.
Coherencelengthsof(010)peaksestimatedbyScherrerequation[13]are11.
5forPIDTBTO-Tand13.
1forPIDTBTO-TT,respectively.
Theimprovementofverticalπ-πstacking,whichisbenecialtochargetransportandreducingbimolecularrecombination,mainlycomesfromthebettermolecularplanarityofthePIDTBTO-TTthanPIDTBTO-Tasevidencedbythetheoreticalcalculation.
2.
5.
PhotovoltaicpropertiesofsinglejunctiondevicesThephotovoltaicpropertiesofbothtwocopolymerswereinvesti-Fig.
2.
DFT-calculatedLUMOandHOMOofthegeometryoptimizedstructuresofanalogousdimersofPIDTBTO-T(left),andPIDTBTO-TT(right).
Y.
Maetal.
NanoEnergy33(2017)313–324316gatedwithaconventionaldevicecongurationofITO/PEDOT:PSS/polymer:PC71BM/PDIN/Al.
Here,PDINwasemployedasthecathodeinterlayerbecauseitcanlowertheworkfunctionoftheelectrode,allowinghigherwork-functionmetals(suchasAl)toactasthecathode[5a].
PC71BMwaschosenastheelectronacceptorduetoitsbroaderandstrongerabsorptioninthevisibleregion,whichiscomplementarytotheabsorptionvalleyofthecopolymersandbenecialforhigheciencyPSCs[14].
Theactivelayerswerepreparedbyspincoatingthepolymer:PC71BMblendsonthetopofPEDOT:PSSlayerwithoutanyfurthertreatment.
ThemeasurementswereperformedundersimulatedAM1.
5G,100mW/cm2illuminationwithanactiveareaof0.
06cm2.
Thecurrentdensity-voltage(J-V)characteristicsofthesePSCsareshowninFig.
5a,andtheparametersofopencircuitvoltage(Voc),short-circuitcurrent(Jsc),llfactor(FF),andPCEaresummar-izedinTable3.
Initially,thedeviceperformancewasoptimizedthroughseveralaspectsasfollows:i)thepolymer:PC71BMblendratio,ii)theproces-singsolvent(CB:DCBratio),iii)theactivelayerthickness(TablesS2–S4,SI).
Undertheoptimaldevicepreparationconditions,thebestperformancedevicebasedonPIDTBTO-TshowsaPCEof6.
40%withVoc=0.
86V,Jsc=12.
36mAcm2,andFF=59.
90%.
Incontrast,thebestperformancedevicebasedonPIDTBTO-TTexhibitsanimprovedPCEof7.
08%withVoc=0.
88V,Jsc=13.
21mAcm2andFF=60.
85%.
TheVocofPIDTBTO-TT-baseddevicewashigherthanthatofPIDTBTO-T-basedcounterpart,whichcanbeattributedtothelower-lyingHOMOenergylevelofPIDTBTO-TT.
SincetheVocofagivenPSCisrelatedtotheenergyleveldierencebetweentheHOMOofthepolymerandtheLUMOofPC71BM.
TheincreasedJscandFFforthePIDTBTO-TT-baseddevicecouldbetheresultofoptimizedmorphologyandhigherholemobilityofthepolymerblend.
TheresultsdemonstratethatinsertingtheTTπ-bridgeintoIDT-BTO-basedpolymersystemisaneectivestrategytoenhanceJscandFFwithoutsacricingVocvaluesofPSCs.
Inordertofurtheroptimizethephotovoltaicperformanceofthetwocopolymersthroughlmmorphologytuning,1,8-diiodooctane(DIO)wasaddedintotheactivelayersolutionspriortothespin-coatingprocess(TableS5,SI).
When0.
7%(v/v)ofDIOwasadded,theVocandJscofthePIDTBTO-TT-baseddevicesincreaseto0.
91Vand14.
77mAcm2,respectively.
Asaconsequence,thebestperformancedevicebasedonPIDTBTO-TTexhibitsanincreasedPCEof8.
15%.
Tothebestofourknowledge,thiseciencyrepresentsthehighestvaluereportedsofarforsingle-junctionPSCsbasedonIDT-containingcopolymers.
Conversely,theadditionofDIO(0.
7%byvolume)tothePIDTBTO-T:PC71BMblendleadstoadrasticdropinPCE(4.
08%),withadecreaseinVoc(0.
84V),Jsc(10.
28mAcm2),andFF(47.
22%).
ThedropinVoccanbeattributedtotheloweredenergyofcharge-separatedstateupontheDIOaddition[15].
AndthedecreasesinJSCandFF,whenDIOwasused,couldberelatedtothemorphologychanges[16].
Toascertaintheaccuracyofthemeasurements,externalquantumeciencies(EQEs)ofthedeviceswithorwithoutDIOadditiveweremeasuredandshowninFig.
5b.
AlltheEQEspectracoverabroadspectralresponserangefrom300to700nm,whichagreewiththeabsorptionspectraofthepolymerblends(PIDTBTO-TT:PC71BMwasusedasanexample,Fig.
S5,SI).
Inthisrange,theaverageEQEvaluesofPIDTBTO-T-andPIDTBTO-TT-baseddeviceswithoutDIOare55%and58%,respectively.
AfteraddingDIO,theaverageEQEvalueschangeto45%and65%forPIDTBTO-T-andPIDTBTO-TT-baseddevices,respectively.
ThechangesinEQEareinagreementwiththecorrespondingchangesinJscaswellasPCEforbothcopolymers.
Atthesametime,theJscvaluescalculatedbyintegratingtheEQEdatawiththeAM1.
5GsolarspectrumareclosetothoseobtainedfromtheJ-Vmeasurementswithin3%mismatches.
Forexample,forthedeviceFig.
3.
TypicaloutputcharacteristicsofOFETsbasedonPIDTBTO-T(a)andPIDTBTO-TT(c);andtransfercharacteristicsofOFETsbasedonPIDTBTO-T(b)andPIDTBTO-TT(d).
Table2Mobilities,on-offcurrentratios,andthresholdvoltagesofOFETsbasedonthecopolymers.
Polymerμ(cm2V1s1)aIon/IobVt(V)cPIDTBTO-T(3.
3±0.
3)*103103(3–10)PIDTBTO-TT(9.
1±0.
1)*103103(4–10)aHolemobilitymeasuredatVD=80V.
bCurrentontooratio.
cThresholdvoltage.
Y.
Maetal.
NanoEnergy33(2017)313–324317basedonPIDTBTO-TTwithameasuredPCEof8.
15%,thecalculatedJscis14.
49mAcm2,onlyslightlylowerthanthemeasuredJscof14.
77mAcm2.
TodeterminetheoriginoftheobservedphotovoltaicperformancedierencesbetweenPIDTBTO-TTandPIDTBTO-Tandtheeectofthesolventadditive(DIO)ontheactivelayer,wemeasuredthechargetransportpropertiesoftheblendlmsbythespacechargelimitedcurrent(SCLC)method.
ItshouldbenotedherethattheOFETdataonlyrepresentthelateralmobilityatcarrierconcentrationgenerallygreaterthanthatinPSCs.
Toquantifythecarriermobilityperpendiculartothesubstrateplane,theSCLCmeasurementswerethereforecarriedout.
Thestructuresofhole-andelectron-onlydevicesareITO/PEDOT:PSS/polymer:PC71BM/MoO3/AuandITO/ZnO/polymer:PC71BM/Ca/Al,re-spectively.
TheholeandelectronmobilitiescalculatedfromtheSCLCmodelaresummarizedinTable3,andthecorrespondingJ–VcurvesunderdarkconditionareshowninFig.
S6(SI).
ForthedeviceswithoutDIO,theholemobilitiesare8.
1*106and1.
7*105cm2V1s1forPIDTBTO-TandPIDTBTO-TT,respectively;andtheelectronmobilitiesare2.
2*105and3.
1*105cm2V1s1forPIDTBTO-TandPIDTBTO-TT,respectively.
Thisexplainsthebetterphotovoltaicperformance,inparticularforthehigherJscandFFvaluesofthePIDTBTO-TT-basedPSCscomparedtothePIDTBTO-T-baseddevices.
AfteraddingDIOastheprocessingadditive,thePIDTBTO-TT:PC71BMlmshowsaslightlyincreasedholemobilityof2.
3*105cm2V1s1,andasimilarelectronmobilityof3.
5*105cm2V1s1.
Whereas,thePIDTBTO-T:PC71BMlmexhibitssignicantlydecreasedholeandelectronmobilitiesof1.
2*106and7.
6*106cm2V1s1,respectively.
ThisobservationisconsistentwiththecorrespondingPCEchangesinTable3.
Thehigherchargecarriermobilityandmorebalancedcarriertransport(reducedμe/μhratioinTable3)ofPIDTBTO-TT-baseddevicethanthoseofPIDTBTO-T-baseddeviceafteraddingDIOaremainreasonsfortheenhancedJscandFFofthecorrespondingdevice.
Tofurtherunderstandthephotovoltaicperformanceofthetwocopolymersaswellastheadditive(DIO)eectontheactivelayer,themorphologiesoftheactivelayerswereinvestigatedbytransmissionelectronmicroscopy(TEM)andatomicforcemicroscopy(AFM).
Fig.
6showstheTEMimagesoftheblendlms.
ItcanbefoundthatPIDTBTO-TandPIDTBTO-TTblendlmswithoutDIOshowsimilarphaseseparationwithdomainsizesof30–50nm.
Thus,theenhancedJscandFFofPIDTBTO-TT-baseddevicesmaybecontributedtothehighermobilityoftheactivelayer.
AftertheadditionofDIO,thelmmorphologiesofPIDTBTO-TandPIDTBTO-TTblendlmsexhibittwodistinctconversions.
InthecaseofthePIDTBTO-Tblend,theadditionofDIOleadstotheformationofapparentaggregateswithadomainsizeofmorethan100nm.
Thiskindofmorphologywillresultininecientexcitonseparationandchargetransport,andthusworsenthephotovoltaicperformanceofthecorrespondingdevice.
Incontrast,theadditionofDIOtothePIDTBTO-TTblendleadstoanimprovedmorphology.
TherelativelybigsizeaggregationsofpolymerandPC71BMdiminishedandabrousnetworkcanbeclearlyobserved.
Suchnetworkisbenecialforexcitonseparationandchargetransport,whichisingoodagreementwiththeaforementionedincreasedPCEandhole/electronmobility.
ThismorphologychangetrendscanalsobeobservedfromtheAFMimages(Fig.
S7,SI).
TheadditionofDIOtothePIDTBTO-Tblendlmleadstolargesizedomains,whicharenotfavourableforthetransportationofexcitontothedonor-acceptorinterface.
However,theadditionofDIOtothePIDTBTO-TTblendlmleadstomoreobviousphaseseparationwhichisbenecialforchargetransportation.
Atthesametime,blendlmsofPIDTBTO-TandPIDTBTO-TTwithPC71BMshowroot-mean-square(r.
m.
s.
)surfaceroughnessesof0.
23and0.
39nm,respectively.
AftertheadditionofDIO,theroughnessofthePIDTBTO-TT:PC71BMlmbarelychanges(0.
38nm),whereasthatforthePIDTBTO-T:PC71BMlmincreasesto2.
32nminagreementwiththeformationoflargeaggregates.
GIWAXSisalsousedtoinvestigatethemolecularpackingandcrystallinityofblendedlmswithandwithoutDIO.
The2DGIWAXSpatternsofthespin-coatedlmsareshowninFig.
S8a–d(SI)andcorresponding1Dline-cutsinthein-planeandout-of-planedirectionsareshowninFig.
S8e(SI).
Ingeneral,increasedout-of-plane(100)peakswithlongercoherencelengthsarefoundforthePIDTBTO-TTFig.
4.
2DGIWAXSpatternsofspin-coatedthinlmsofPIDTBTO-T(a)andPIDTBTO-TT(b),andthecorresponding1Dline-cutsinthein-planeandout-of-planedirections(c).
Y.
Maetal.
NanoEnergy33(2017)313–324318blendlmwithDIOorwithoutDIOincomparisonwiththePIDTBTO-Tblendlm,suggestingthebettercrystallinityoftheformercopoly-mer.
TheseresultsareinagreementwiththeimproveddeviceperformanceforPIDTBTO-TTincomparisonwithPIDTBTO-T.
However,the2DGIWAXSresultdoesnotrevealsignicantadditiveeectonthecrystallinityandthepackingdistanceforbothcopolymerblends.
2.
6.
PhotovoltaicpropertiesoftandemdevicesToinvestigatetheapplicationofthesewidebandgapcopolymersinmulti-junctionPSCs,double-junctiontandemcellswithadevicestructureofITO/PEDOT:PSS/PIDTBTO-TT:PC71BM/PDIN:PFN/Al/MoO3/PTB7-Th:PC71BM/PDIN/Al(Fig.
7a)werefabricatedasanexample.
ThechemicalstructuresofPTB7-Th,PDINandPFNareshowninFig.
S1(SI).
Here,thelowbandgappolymerPTB7-Thwasselectedasthetopcellactivematerialbecauseithasalowbandgapof1.
58eVwithastrongabsorptionbandintherangeof550–780nm(Fig.
7b)[2c–e],whichissomewhatcomplementarytotheabsorptionproleofPIDTBTO-TT.
AsshowninFig.
7a,PDINblendedwithPFNwas,forthersttime,usedastheelectrontransportlayer(ETL)forbottomsub-cell,andmolybdenumoxide(MoO3)wasemployedastheholetransportlayerforthetopsub-cell.
Meanwhile,anultra-thinAl(1nm)lmwasinsertedbetweenthePDIN:PFNandMoO3layerstoestablishanohmiccontact.
ThePDIN:PFNETLwouldhavecombinedadvantagesofthePDINandPFNlayers.
Forexample,thepoorlmformingpropertiesofPDINcanbeimprovedbyblendingwiththePFNcopolymer.
Atthesametime,thepresenceofPDINmayprotectPFNfrombeingdamagedbychlorinatedsolvents.
Moredetailedinvestiga-tiononthisblendedETLisstillunderwayandwillbepublishedinduecourse.
ThetandemdeviceswereoptimizedbyvaryingtheETLintheinterconnectinglayerandtheactivelayerthicknesses.
TheresultsshowthatthedevicesusingPDIN:PFNastheETLandtheactivelayerwiththicknessesof141and85nmforbottomandtopcellsgivethehighestPCEs(Figs.
S9–S10,TablesS6–S7,SI).
J-VcharacteristicsofthebestperformancetandemPSCsandcorrespondingsingle-junctionrefer-encesub-cellsareshowninFig.
7c,andthedetailedperformanceparametersaresummarizedinTable4.
ThebestperformancetandemcellshowsahighPCEof11.
15%withJsc=9.
68mAcm2,Voc=1.
70V,FF=67.
79%.
Withthesameactivelayers(thesamethicknessaswell),thereferencebottom(PIDTBTO-TT:PC71BM)andtop(PTB7-Th:PC71BM)sub-cellsshowmoderatePCEsof7.
34%and7.
86%,respectively.
TheircorrespondingEQEspectraareshowninFig.
S11(SI)withsmallspectralmismatches(720nm)andabandpasslter(380–420nm),respectively.
Thebeamsizeofthelightsourceisslightlysmallerthanthedeviceareatoavoiderrors.
4.
5.
OFETfabricationandcharacterizationTop-contact/bottom-gateOFETdeviceswerefabricatedusingSi/SiO2substrateswhereSiandSiO2wereusedasthegateelectrodeandgatedielectric,respectively.
Thesubstratesweresubjectedtocleaningbyultrasonicationinpiranhasolution(H2SO4/H2O2=3/1),deionizedwater,acetone,andisopropanol,anddriedinoven.
Tooptimizedeviceperformance,thesubstratesweremodiedwithoctadecyltrimethox-ysilane(OTS)toformaself-assembledmonolayer.
Thinlmsofthepolymersweredepositedonthetreatedsubstratesbyspincoatingapolymersolution(6mg/mL)inDCBundernitrogen.
Afterpolymerthinlmdeposition,about40nmthickgoldelectrodewasdepositedassourceanddraincontactsusingashadowmask.
TheOFETdeviceshadachannellength(L)of300mandachannelwidth(W)of6mm.
TheevaluationsoftheOFETswerecarriedoutinambientatmosphereusinganAgilent4155Csemiconductorparameteranalyzeronaprobestation.
Thecarriermobility,μ,wascalculatedfromthedatainthesaturatedregionaccordingtotheequation:(Id)sat=(W/2L)μCi(Vg–Vth)2,where(Id)satisthedraincurrentinthesaturatedregion,WandLarethesemiconductorchannelwidthandlength,respectively,Ci(Ci=10nF)isthecapacitanceofthegatedielectriclayer,andVgandVtharethegatevoltageandthethresholdvoltage,respectively.
Thesaturationregionmobilitieswerecalculatedfromthetransferchar-acteristicsoftheFETsusingtheslopederivedfromthesquarerootoftheabsolutevalueofthecurrentasafunctionofgatevoltagebetween80and20V.
4.
6.
Hole-andelectron-onlydevicefabricationandcharacterizationHole-onlydiodeswerefabricatedonITOcoatedglasswithaPEDOT:PSSbottomcontactandaMoO3/Autopcontact.
Electron-onlydiodeswerefabricatedonITOcoatedglasswithaZnObottomcontactandaCa/Altopcontact.
TheBHJlmswerepreparedusingthesamemethodasthatusedintheoptimalsolarcellfabrication.
Deviceareaswere6mm2.
Thecurrentdensity(J)wasmeasuredbyaKeithley2440sourcemeasurementunit.
TheSCLCholemobilitieswerecalculatedaccordingtothefollowingequation:[20]JεεμVL=98r023whereε0isthepermittivityoffreespace(8.
85*1012Fm1),εristhedielectricconstantofthepolymer(assumedtobe3,whichisatypicalvalueforconjugatedpolymers),μisthecarriermobility,Visthevoltagedropacrossthedevice(V=VapplVbi,whereVapplistheappliedvoltagetothedevice,andVbiisthebuilt-involtageduetothedierenceinworkfunctionofthetwoelectrodes),andListhepolymerthickness.
ThethicknessofthelmwasmeasuredbyaBrukerDektakXTsurfaceprolometer.
4.
7.
GIWAXScharacterizationGIWAXSmeasurementswereperformedatbeamline7.
3.
3attheAdvancedLightSource(ALS).
SampleswerepreparedonSisubstratesusingidenticalpolymersolutionsasthoseusedinFETorOPVdevices.
The10keVX-raybeamwasincidentatagrazingangleof0.
11–0.
15°,whichmaximizedthescatteringintensityfromthesamples.
ThescatteredX-raysweredetectedusingaDectrisPilatus1Mphotoncountingdetector.
4.
8.
SynthesisofPIDTBTO-TIDT(0.
24g,0.
19mmol)andBTO-T(0.
14g,0.
19mmol)weredissolvedintoluene(20mL).
Themixturewaspurgedwithnitrogenfor1hatroomtemperature.
AftertheadditionofPd2(dba)3(4mg)andP(o-tolyl)3(10mg),themixturewasheatedtoreuxfor12hunderanitrogenatmosphere.
Then,adropof2-tributylstannylthiophenewasaddedtothemixtureandreactedfor3h.
Finally,twodropsof2-bromothiophenewasaddedtothemixtureandreactedovernighttocompletetheend-cappingreaction.
Thereactionmixturewasprecipi-tatedintomethanolandltered.
Thecollectedprecipitatewassub-jectedtoSoxhletextractionwithmethanol,acetone,hexane,andchloroformfor24heach.
Thechloroformextractwasconcentrated,andthenprecipitatedintomethanol.
Thetargetpolymerwascollectedbyltrationanddriedinvacuoat50°Covernighttogiveablacksolid(0.
25g,89%).
1HNMR(CDCl3,400MHz,ppm):8.
49(br,2H),7.
45(br,2H),7.
30(br,4H),7.
24(br,8H),7.
14(br,8H),4.
17(br,4H),2.
61(br,8H),1.
98(br,4H),1.
64(m,8H),1.
51(m,4H),1.
34(m,40H),0.
90(m,18H).
GPC:Mn=97.
5kDa,PDI=2.
5.
4.
9.
SynthesisofPIDTBTO-TTIDT(0.
31g,0.
25mmol)andBTO-TT(0.
21g,0.
25mmol)weredissolvedintoluene(20mL).
Themixturewaspurgedwithnitrogenfor1hatroomtemperature.
Aftertheadditionof14.
5mgofPd(PPh3)4,themixturewasheatedtoreuxfor9hunderanitrogenatmosphere.
Then,adropof2-tributylstannylthiophenewasaddedtothemixtureandreactedfor3h.
Finally,twodropsof2-bromothiophenewasaddedtothemixtureandreactedovernighttocompletetheend-cappingreaction.
Thereactionmixturewasprecipitatedintomethanolandltered.
ThecollectedprecipitatewassubjectedtoSoxhletextractionwithmethanol,acetone,hexane,dichloromethaneandchloroformforY.
Maetal.
NanoEnergy33(2017)313–32432224heach.
Thechloroformextractwasconcentratedandthenpre-cipitatedintomethanol.
Thetargetpolymerwascollectedbyltrationanddriedinvacuoat50°Covernighttogiveablacksolid(0.
28g,72%).
1HNMR(CDCl3,400MHz,ppm):8.
84(br,2H),7.
45(br,2H),7.
41(br,4H),7.
25-7.
13(m,16H),4.
18(br,4H),2.
61(br,8H),2.
03(br,4H),1.
59(m,8H),1.
51(m,4H),1.
33(m,4H),0.
91(m,18H).
GPC:Mn=119.
9kDa,PDI=1.
8.
AcknowledgementsThisworkwassupportedbytheNationalNaturalScienceFoundationofChina(Nos.
U1605241,61325026,51561165011),theStrategicPriorityResearchProgramoftheChineseAcademyofSciences(No.
XDB20000000),andtheCAS/SAFEAInternationalPartnershipProgramforCreativeResearchTeams.
TheGIWAXScharacterizationissupportedbytheDirector,OceofScience,OceofBasicEnergySciences,oftheU.
S.
DepartmentofEnergyunderContractNo.
DE-AC02-05CH11231.
AppendixA.
SupplementarymaterialSupplementarydataassociatedwiththisarticlecanbefoundintheonlineversionathttp://dx.
doi.
org/10.
1016/j.
nanoen.
2017.
01.
050.
References[1](a)J.
Chen,Y.
Cao,Acc.
Chem.
Res.
42(2009)1709–1718(b)G.
Li,R.
Zhu,Y.
Yang,Nat.
Photonics6(2012)153–161(c)Y.
Li,Acc.
Chem.
Res.
45(2012)723–733(d)L.
Ye,S.
Q.
Zhang,L.
J.
Huo,M.
J.
Zhang,J.
H.
Hou,Acc.
Chem.
Res.
47(2014)1595–1603(e)J.
-S.
Wu,S.
-W.
Cheng,Y.
-J.
Cheng,C.
-S.
Hsu,Chem.
Soc.
Rev.
44(2015)1113–1154(f)L.
Lu,T.
Zheng,Q.
Wu,A.
M.
Schneider,D.
Zhao,L.
Yu,Chem.
Rev.
115(2015)12666–12731(g)W.
Li,K.
H.
Hendriks,M.
M.
Wienk,R.
A.
J.
Janssen,Acc.
Chem.
Res.
49(2016)78–85.
[2](a)Y.
Liu,J.
Zhao,Z.
Li,C.
Mu,W.
Ma,H.
Hu,K.
Jiang,H.
Lin,H.
Ade,H.
Yan,Nat.
Commun.
5(2014)5293(b)Y.
Liu,Z.
A.
Page,T.
P.
Russell,T.
Emrick,Angew.
Chem.
Int.
Ed.
54(2015)11485–11489(c)X.
Ouyang,R.
Peng,L.
Ai,X.
Zhang,Z.
Ge,Nat.
Photonics9(2015)520–524(d)J.
-D.
Chen,C.
Cui,Y.
-Q.
Li,L.
Zhou,Q.
-D.
Ou,C.
Li,Y.
Li,J.
-X.
Tang,Adv.
Mater.
27(2015)1035–1041(e)Z.
C.
He,B.
Xiao,F.
Liu,H.
B.
Wu,Y.
L.
Yang,S.
Xiao,C.
Wang,T.
P.
Russell,Y.
Cao,Nat.
Photonics9(2015)174–179(f)J.
B.
You,L.
T.
Dou,K.
Yoshimura,T.
Kato,K.
Ohya,T.
Moriarty,K.
Emery,C.
-C.
Chen,J.
Gao,G.
Li,Y.
Yang,Nat.
Commun.
4(2013)1446(g)N.
Li,C.
J.
Brabec,EnergyEnviron.
Sci.
8(2015)2902–2909(h)A.
R.
b.
M.
Yuso,D.
Kim,H.
P.
Kim,F.
K.
Shneider,W.
J.
daSilva,J.
Jang,EnergyEnviron.
Sci.
8(2015)303–316.
[3](a)F.
Guo,N.
Li,F.
W.
Fecher,N.
Gasparini,C.
O.
R.
Quiroz,C.
Bronnbauer,Y.
Hou,V.
V.
Radmilovic,V.
R.
Radmilovic,E.
Spiecker,K.
Forberich,C.
J.
Brabec,Nat.
Commun.
6(2015)7730(b)H.
Q.
Zhou,Y.
Zhang,C.
K.
Mai,S.
D.
Collins,G.
C.
Bazan,T.
Q.
Nguyen,A.
J.
Heeger,Adv.
Mater.
27(2015)1767–1773(c)K.
Zhang,K.
Gao,R.
Xia,Z.
Wu,C.
Sun,J.
Cao,L.
Qian,W.
Li,S.
Liu,F.
Huang,X.
Peng,L.
Ding,H.
-L.
Yip,Y.
Cao,Adv.
Mater.
28(2016)4817–4823(d)O.
Adebanjo,B.
Vaagensmith,Q.
Qiao,J.
Mater.
Chem.
A2(2014)10331–10349(e)N.
Li,D.
Baran,K.
Forberich,F.
Machui,T.
Ameri,M.
Turbiez,M.
Carrasco-Orozco,M.
Drees,A.
Facchetti,F.
C.
Krebs,C.
J.
Brabec,EnergyEnviron.
Sci.
6(2013)3407–3413(f)J.
Y.
Kim,K.
Lee,N.
E.
Coates,D.
Moses,T.
Q.
Nguyen,M.
Dante,A.
J.
Heeger,Science317(2007)222–225(g)C.
-Y.
Chang,L.
Zuo,H.
-L.
Yip,C.
-Z.
Li,Y.
Li,C.
-S.
Hsu,Y.
-J.
Cheng,H.
Chen,A.
K.
Y.
Jen,Adv.
EnergyMater.
4(2014)1301645(h)Z.
Zheng,S.
Zhang,J.
Zhang,Y.
Qin,W.
Li,R.
Yu,Z.
Wei,J.
Hou,Adv.
Mater.
28(2016)5133–5138(i)L.
Dou,J.
You,J.
Yang,C.
-C.
Chen,Y.
He,S.
Murase,T.
Moriarty,K.
Emery,G.
Li,Y.
Yang,Nat.
Photonics6(2012)180–185.
[4](a)Y.
Ma,Z.
Kang,Q.
Zheng,J.
Mater.
Chem.
A5(2017).
http://dx.
doi.
org/10.
1039/C6TA09325F(b)S.
C.
Price,A.
C.
Stuart,L.
Yang,H.
Zhou,W.
You,J.
Am.
Chem.
Soc.
133(2011)4625–4631(c)Y.
Dong,X.
W.
Hu,C.
H.
Duan,P.
Liu,S.
J.
Liu,L.
Y.
Lan,D.
C.
Chen,L.
Ying,S.
J.
Su,X.
Gong,F.
Huang,Y.
Cao,Adv.
Mater.
25(2013)3683–3688(d)C.
Cabanetos,A.
ElLabban,J.
A.
Bartelt,J.
D.
Douglas,W.
R.
Mateker,J.
M.
J.
Frechet,M.
D.
McGehee,P.
M.
Beaujuge,J.
Am.
Chem.
Soc.
135(2013)4656–4659(e)Y.
Ma,Q.
Zheng,L.
Wang,D.
Cai,C.
Tang,M.
Wang,Z.
Yin,S.
-C.
Chen,J.
Mater.
Chem.
A2(2014)13905–13915(f)J.
W.
Jung,F.
Liu,T.
P.
Russell,W.
H.
Jo,Adv.
EnergyMater.
5(2015)1503902(g)M.
Wang,D.
Cai,Z.
Yin,S.
-C.
Chen,C.
-F.
Du,Q.
Zheng,Adv.
Mater.
28(2016)3359–3365(f)M.
Wang,Z.
Wang,W.
Ma,S.
-C.
Chen,Q.
Zheng,Adv.
Electron.
Mater.
2(2016)1600340.
[5](a)Z.
-G.
Zhang,B.
Qi,Z.
Jin,D.
Chi,Z.
Qi,Y.
Li,J.
Wang,EnergyEnviron.
Sci.
7(2014)1966–1973(b)Z.
Yin,J.
Wei,Q.
Zheng,Adv.
Sci.
3(2016)1500362.
[6](a)X.
Wang,Y.
Sun,S.
Chen,X.
Guo,M.
Zhang,X.
Li,Y.
Li,H.
Wang,Macromolecules45(2012)1208–1216(b)X.
Guo,M.
J.
Zhang,L.
J.
Huo,F.
Xu,Y.
Wu,J.
H.
Hou,J.
Mater.
Chem.
22(2012)21024–21031(c)J.
J.
Intemann,K.
Yao,Y.
-X.
Li,H.
-L.
Yip,Y.
-X.
Xu,P.
-W.
Liang,C.
-C.
Chueh,F.
-Z.
Ding,X.
Yang,X.
Li,Y.
Chen,A.
K.
Y.
Jen,Adv.
Funct.
Mater.
24(2014)1465–1473(d)G.
Zuo,Z.
Li,M.
Zhang,X.
Guo,Y.
Wu,S.
Zhang,B.
Peng,W.
Wei,J.
Hou,Polym.
Chem.
5(2014)1976–1981.
[7](a)M.
H.
Chen,J.
Hou,Z.
Hong,G.
Yang,S.
Sista,L.
M.
Chen,Y.
Yang,Adv.
Mater.
21(2009)4238–4242(b)Q.
Zheng,B.
J.
Jung,J.
Sun,H.
E.
Katz,J.
Am.
Chem.
Soc.
132(2010)5394–5404(c)K.
Li,Z.
Li,K.
Feng,X.
Xu,L.
Wang,Q.
Peng,J.
Am.
Chem.
Soc.
135(2013)13549–13557.
[8](a)R.
P.
Qin,W.
W.
Li,C.
H.
Li,C.
Du,C.
Veit,H.
F.
Schleiermacher,M.
Andersson,Z.
S.
Bo,Z.
P.
Liu,O.
Inganas,U.
Wuerfel,F.
L.
Zhang,J.
Am.
Chem.
Soc.
131(2009)14612–14613(b)L.
Wang,D.
Cai,Z.
Yin,C.
Tang,S.
-C.
Chen,Q.
Zheng,Polym.
Chem.
5(2014)6847–6856(c)Y.
Ma,Q.
Zheng,Z.
Yin,D.
Cai,S.
-C.
Chen,C.
Tang,Macromolecules46(2013)4813–4821.
[9]C.
-P.
Chen,S.
-H.
Chan,T.
-C.
Chao,C.
Ting,B.
-T.
Ko,J.
Am.
Chem.
Soc.
130(2008)12828–12833.
[10](a)Y.
Zhang,J.
Zou,H.
-L.
Yip,K.
-S.
Chen,D.
F.
Zeigler,Y.
Sun,A.
K.
Y.
Jen,Chem.
Mater.
23(2011)2289–2291(b)M.
Zhang,X.
Guo,X.
Wang,H.
Wang,Y.
Li,Chem.
Mater.
23(2011)4264–4270(c)X.
Guo,M.
Zhang,J.
Tan,S.
Zhang,L.
Huo,W.
Hu,Y.
Li,J.
Hou,Adv.
Mater.
24(2012)6536–6541(d)H.
Bronstein,J.
M.
Frost,A.
Hadipour,Y.
Kim,C.
B.
Nielsen,R.
S.
Ashraf,B.
P.
Rand,S.
Watkins,I.
McCulloch,Chem.
Mater.
25(2013)277–285(e)M.
Wang,X.
Hu,L.
Liu,C.
Duan,P.
Liu,L.
Ying,F.
Huang,Y.
Cao,Macromolecules46(2013)3950–3958(f)D.
Liu,L.
Sun,Z.
Du,M.
Xiao,C.
Gu,T.
Wang,S.
Wen,M.
Sun,R.
Yang,RSCAdv.
4(2014)37934–37940(g)L.
Zuo,C.
-Y.
Chang,C.
-C.
Chueh,S.
Zhang,H.
Li,A.
K.
Y.
Jen,H.
Chen,EnergyEnviron.
Sci.
8(2015)1712–1718.
[11](a)Y.
Lin,J.
Wang,Z.
G.
Zhang,H.
Bai,Y.
Li,D.
Zhu,X.
Zhan,Adv.
Mater.
27(2015)1170–1174(b)Y.
Lin,Q.
He,F.
Zhao,L.
Huo,J.
Mai,X.
Lu,C.
-J.
Su,T.
Li,J.
Wang,J.
Zhu,Y.
Sun,C.
Wang,X.
Zhan,J.
Am.
Chem.
Soc.
138(2016)2973–2976.
[12](a)A.
A.
Y.
Guilbert,J.
M.
Frost,T.
Agostinelli,E.
Pires,S.
Lilliu,J.
E.
Macdonald,J.
Nelson,Chem.
Mater.
26(2014)1226–1233(b)A.
Hexemer,W.
Bras,J.
Glossinger,E.
Schaible,E.
Gann,R.
Kirian,A.
MacDowell,M.
Church,B.
Rude,H.
Padmore,in:Proceedingsofthe14thInternationalConferenceonSmall-AngleScattering(SAS09),Oxford,England,Sep13–18,2009.
[13](a)D.
-M.
Smilgies,J.
Appl.
Crystallogr.
46(2013)286(b)R.
Noriega,J.
Rivnay,K.
Vandewal,F.
P.
Koch,N.
Stingelin,P.
Smith,M.
F.
Toney,A.
Salleo,Nat.
Mater.
12(2013)1038–1044.
[14]M.
M.
Wienk,J.
M.
Kroon,W.
J.
H.
Verhees,J.
Knol,J.
C.
Hummelen,P.
A.
vanHal,R.
A.
J.
Janssen,Angew.
Chem.
Int.
Ed.
115(2003)3493–3497.
[15]D.
D.
Nuzzo,A.
Aguirre,M.
Shahid,V.
S.
Gevaerts,S.
C.
Meskers,R.
A.
Janssen,Adv.
Mater.
22(2010)4321–4324.
[16](a)C.
B.
Nielsen,R.
S.
Ashraf,N.
D.
Treat,B.
C.
Schroeder,J.
E.
Donaghey,A.
J.
P.
White,N.
Stingelin,I.
McCulloch,Adv.
Mater.
27(2015)948–953(b)H.
Zhong,Z.
Li,F.
Deledalle,E.
C.
Fregoso,M.
Shahid,Z.
Fei,C.
B.
Nielsen,N.
Yaacobi-Gross,S.
Rossbauer,T.
D.
Anthopoulos,J.
R.
Durrant,M.
Heeney,J.
Am.
Chem.
Soc.
135(2013)2040–2043(c)N.
Wang,Z.
Chen,W.
Wei,Z.
Jiang,J.
Am.
Chem.
Soc.
135(2013)17060–17068.
[17](a)A.
Martinez-Otero,Q.
Liu,P.
Mantilla-Perez,M.
M.
Bajo,J.
Martorell,J.
Mater.
Chem.
A3(2015)10681–10686(b)Y.
Liu,C.
-C.
Chen,Z.
Hong,J.
Gao,Y.
Yang,H.
Zhou,L.
Dou,G.
Li,Sci.
Rep.
3(2013)3356.
[18]J.
-H.
Kim,J.
B.
Park,F.
Xu,D.
Kim,J.
Kwak,A.
C.
Grimsdale,D.
-H.
Hwang,EnergyEnviron.
Sci.
7(2014)4118–4131.
[19]S.
Zeng,L.
Yin,C.
Ji,X.
Jiang,K.
Li,Y.
Li,Y.
Wang,Chem.
Commun.
48(2012)10627–10629.
[20]G.
G.
Malliaras,J.
R.
Salem,P.
J.
Brock,C.
Scott,Phys.
Rev.
B58(1998)13411–13414.
Y.
Maetal.
NanoEnergy33(2017)313–324323YunlongMareceivedhisB.
S.
degreefromMinjiangUniversityin2009,andM.
S.
degreeinmaterialsscienceandengineeringfromFujianNormalUniversityin2012.
HeiscurrentlypursuinghisPh.
D.
underthesupervisionofProf.
QingdongZheng.
Hisresearchinterestsincludethesynthesisoforganicsemiconductingmaterialsforpolymersolarcells.
Shan-CiChenreceivedhisB.
S.
degreeinchemistryfromXiamenUniversityin2005andaPh.
D.
degreeinphysicalchemistryfromFujianInstituteofResearchontheStructureofMatter,ChineseAcademyofSciencein2010.
HeisnowanassociateprofessorinProf.
QingdongZheng'sgroupattheFujianInstituteofResearchontheStructureofMatter,ChineseAcademyofSciences.
Dr.
Chen'sinterestinvolvesdesign,synthesis,andcharacterizationsofmateri-alsforvariousapplicationsincludingorganiceld-eecttransistors,organicsolarcellsetc.
ZaiyuWangreceivedhisB.
EnginMaterialsScience&EngineeringfromXi'anJiaotongUniversityin2015andjoinedProf.
Ma'sgroupthesameyear.
HeiscurrentlyworkingonthemorphologycharacterizationoforganicsolarcellsatXi'anJiaotongUniversityasagraduatestudent.
WeiMaobtainedhisPh.
D.
inChemicalPhysicsfromtheUniversityofPierreMarieCurie(Paris6,France)in2010beforemovingtotheEcoleNormaleSuperieure(Paris),France(2010–1011)andNorthCarolinaStateUniversity(NCSU)asPostdocFellow.
HejoinedtheSchoolofMaterialsScienceEngineeringatXi'anJiaotongUniversityin2014asaProfessor.
Hisresearchinterestsincludetheuseofsoft/hardX-raybasedtechniquestounraveltherelationshipbetweenlmmicrostructureanddeviceperformance.
JinyunWangreceivedherB.
S.
degreeineducationofchemistryfromFujianNormalUniversity,Chinain2006.
AndshegottheM.
S.
andPh.
DdegreesintheoreticalandcomputationalchemistryfromFujianInstituteofResearchontheStructureofMatter(FJIRSM),ChineseAcademyofSciencesin2008and2011,respectively.
Afterthat,sheservicedasanAssistantProfessorintheStateKeyLaboratoryofStructuralChemistryandChemistryDivisionatFJIRSM.
Herresearchprojectisrelatedtothetheoreticalstudyofthephotophysicalandelectron-trans-portpropertiesofthemetallorganiccomplexes.
ZhigangYinreceivedhisbachelordegreefromXi'anTechnologicalUniversityin2008andhismaster'sdegreeinmaterialsphysicsandchemistryfromFuzhouUniversityin2011.
HethenjoinedtheFujianInstituteofResearchontheStructureofMatter,ChineseAcademyofSciences(CAS),andbecameanassociateprofessorin2016.
Hisresearchinterestsincludeoptoelectronicmaterials,energydevices,andinterfacialsciences.
ChangquanTangreceivedhisB.
S.
degreefromJiangxiNormalUniversityin2007andM.
S.
degreeinorganicchemistryfromCentralSouthUniversityinChinain2010.
HeiscurrentlypursuinghisPh.
D.
intheUniversityofChineseAcademyofSciences.
Hisresearchinterestsfocusonthesynthesisandapplicationsofphoton-activemateri-als.
DongdongCaireceivedhisbachelordegreeandMaster'sdegreefromFujianNormalUniversityin2009and2012,respectively.
HethenjoinedtheFujianInstituteofResearchonthestructureofMatter,ChineseAcademyofSciences.
Hisresearchinterestsincludeorganicsemicon-ductormaterials,organicsolarcells,andorganiceldeecttransistors.
QingdongZhengreceivedhisB.
S.
degreeinnechemi-calsandhisM.
S.
degreeinappliedchemistryfromEastChinaUniversityofScienceandTechnology,Chinain1998and2001,respectively.
HeobtainedhisPh.
D.
degreeinchemistryfromtheStateUniversityofNewYorkatBualo,USAin2005.
AftercarryingouthispostdoctoralresearchattheJohnsHopkinsUniversity,hejoinedtheFujianInstituteofResearchontheStructureofMatter,ChineseAcademyofSciences,andbecameaprofessorin2010.
Hismaininterestsfocusonmultifunctionalmolecularmateri-alsanddevicesandinparticularontheeldsofsemi-conductingmaterialsandrelatedinterfacialmaterialsfororganicsolarcells.
Hehaspublishedmorethan80peer-reviewedpapers.
HeisservingasAssociateeditorofRSCAdvancesandastopicaleditorofChineseOpticsLetters.
Y.
Maetal.
NanoEnergy33(2017)313–324324
elsevier.
com/locate/nanoenFullpaperIndacenodithiophene-basedwidebandgapcopolymersforhighperformancesingle-junctionandtandempolymersolarcellsYunlongMaa,b,Shan-CiChena,ZaiyuWangc,WeiMac,JinyunWanga,ZhigangYina,b,ChangquanTanga,b,DongdongCaia,QingdongZhenga,aStateKeyLaboratoryofStructuralChemistry,FujianInstituteofResearchontheStructureofMatter,ChineseAcademyofSciences,155YangqiaoWestRoad,Fuzhou,Fujian350002,ChinabUniversityofChineseAcademyofSciences,19YuquanRoad,Beijing100049,ChinacStateKeyLaboratoryforMechanicalBehaviorofMaterials,Xi'anJiaotongUniversity,Xi'an710049,ChinaARTICLEINFOKeywords:IndacenodithiopheneTandemdevicesElectrontransportlayerFibrousnetworkPolymersolarcellsABSTRACTTwowidebandgapcopolymersbasedonbulkyindacenodithiophene(IDT)andalkoxylatedbenzothiadiazoleunits(PIDTBTO-TandPIDTBTO-TT)withthethiopheneorthieno[3,2-b]thiophene(TT)π-bridgearedesignedandsynthesized.
Theeectofπ-bridgeontheπ-πpacking,optical,carriertransport,nano-sizedphaseseparationandphotovoltaicpropertiesofthecopolymersareinvestigatedindepth.
IncomparisonwiththePIDTBTO-T-basedcounterpart,thebestperformancesolarcellbasedonPIDTBTO-TTexhibitsahigherpowerconversioneciency(PCE)of8.
15%whichismainlyattributedtotheformationofabrousnetworkfortheactivelayerbasedonPIDTBTO-TT.
Furthermore,whenanovelhybridelectrontransportlayer(PDIN:PFN)isintroducedintoatandemsolarcellusingthePIDTBTO-TT-baseddeviceandaPTB7-Th-baseddeviceasthebottomandtopcellcomponents,respectively,theresultingsolarcellexhibitsanoutstandingPCEof11.
15%withalargeopencircuitvoltageof1.
70V.
Tothebestofourknowledge,thePCEsof8.
15%and11.
15%arethehighestvaluesreportedtodateforthesingle-junctionandtandemsolarcellsusingIDT-basedcopolymers,respectively.
Ourresultsdemonstratethattheπ-bridgemodulationiseectiveinadjustingthechargecarriermobilityandphotovoltaicperformanceofIDT-basedwidebandgapcopolymersforsingle-junctionandtandemdevices.
1.
IntroductionPolymersolarcells(PSCs)usingp-typeconjugatedpolymersandn-typefullerenederivativeshaveattractedincreasingattentionduetotheiradvantagesoflowcost,lightweight,andgoodexibility[1].
Inthepastdecade,extensiveeortshavebeendevotedtodevelopingnewp-typelowbandgapconjugatedpolymersforthephotovoltaicapplication.
Asaresult,signicantprogresseshavebeenmadeinthiseld,andpowerconversioneciencies(PCEs)over10%havebeenachievedforbothsingle-junctionandtandemPSCs[2].
Forsingle-junctionPSCsbasedonlowbandgapcopolymers,itisachallengetosimultaneouslyimprovetheshortcircuitcurrentdensity(JSC)andopencircuitvoltage(VOC)valuesbecausethereisatrade-obetweenhighcurrentandlargevoltagevalues.
Asaresult,amulti-junctiondevicewithatandemstructureisdesignedasanalternativetobroadenthelight-harvestingspectraandtoincreasetheVOCvaluesofPSCs.
Foratandemdevice,twocellswithbulk-heterojunction(BHJ)layershavingcomplementaryabsorptionspectraareusuallyneeded,whereabottomcellutilizesawidebandgapmaterialandatopcellusesalowbandgapmaterial.
AlthoughthemaximumPCEupto15%couldbeexpectedaccordingtomodelcalculations[3],bynow,thereareonlyacoupleexamplesofdouble-junctiontandemPSCswithecienciesover11%[3b,c,h],whichismainlyattributedtothelackofsuitablepairofabsorbingmaterialswithdierentbandgapsandcomplementaryabsorptionspectra.
Specically,manyhighperformancelowbandgapcopolymershavebeenreportedinthepastdecade[2].
However,relativelyfewerhighperformancewidebandgapcopolymersareavailablefortandemdevices[4],andmostofpolymertandemdeviceshavetousepoly(3-hexylthiophene)(P3HT,1.
90eVbandgap,HOMO=5.
10eV)astheshort-wavelengthabsorbingmaterial,whichexhibitsarelativelylowVOC(whenblendedwithPC71BM)[2f,3d–f].
Anotherchallengeforpolymertandemdevicesisrelatedtothefactthatthereareonlyfewinterfaciallayersavailabletoconnectdierentsubcellseciently[2e,5].
Therefore,bothecientsolution-processedinterfaciallayersandnewwidebandgapmaterialsareneededforhigheciencytandemsolarcells.
http://dx.
doi.
org/10.
1016/j.
nanoen.
2017.
01.
050Received15December2016;Receivedinrevisedform17January2017;Accepted24January2017Correspondingauthor.
E-mailaddress:qingdongzheng@fjirsm.
ac.
cn(Q.
Zheng).
NanoEnergy33(2017)313–324Availableonline25January20172211-2855/2017ElsevierLtd.
Allrightsreserved.
MARKIndesigninghighperformancewidebandgappolymers,awidelyadoptedstrategyistoalternatesuitableelectron-rich(donor,D)andelectron-decient(acceptor,A)buildingblocksinthepolymerback-bone.
Asbothdonorandacceptorunitsareconsistedofplanararomaticringswithlittleconformationexibility,theconnectionbetweendonorandacceptorunitsbecomesveryimportantindeter-miningthebackboneplanaritywhichsubsequentlyaectstheenergylevels,bandgapsandchargetransportpropertiesoftheresultingD-Acopolymers.
Inthepast10years,in-depthstudiesontheconnectionortheπ-bridgebetweendonorandacceptorunitsaremoreorlessneglectedinspiteofmanydonororacceptorunitsdeveloped[1,6].
Eectiveπ-bridgemodulationstrategiesshouldconsiderthechemicalstructuredierenceofthegivenalternatingdonorandacceptorunits.
Forthecopolymersoriginatedfrombothve-membered-ring-baseddonorandacceptorunits,usuallynoπ-bridgeisneeded.
However,forcopolymersfromasix-memberedring-basedunit(suchasbenzothia-diazole,BT),asuitableve-memberedring-basedπ-bridgeisrequiredanditwillplayanimportantroleindeterminingtheperformanceoftheresultingcopolymers.
π-Bridgessuchasthiophene,bithiophene,thieno[3,2-b]thiophene(TT),andotherve-membered-ring-basedaromaticunitscanbeusedforcopolymers[1g,4a,6,7].
Amongtheseπ-bridges,TTstandsoutasanexcellentcandidateduetoitslinearandextendedbackbonestructurewhichmayreducethetorsionalanglebetweentwoplanaralternatingunitswhilenotgreatlydisturbingtheelectrontransferbetweendonorandacceptorunits.
Withtheafore-mentionedconsideration,theincorporationofTTasaπ-bridgeintocopolymerswithsomeknowndonorandacceptorunitsmayleadtopolymerswithunexpectedperformance,especiallyinthecaseofbulkyunit-basedcopolymers.
5,6-Bis(alkoxy)benzo[1,2,5]thiadiazole(BTO)isatypicalacceptorunitforwidebandgapcopolymers[8a].
Wepreviouslyexploredseveralseriesofcopolymersusingdierentlad-der-typearomaticunitsasdonorsandBTOunitsasacceptors[4e,8b,c].
Amongthem,ahighPCEof7.
26%withaVocof0.
94Vwasachievedfromthealternatingcopolymerbasedondiindenocarbazoleand5,6-bis(butoxy)benzo[c][1,2,5]thiadiazoleunits[8b].
Indacenodithiophene(IDT)isaladder-typedonorunitfeaturingacoplanarandextendedladder-typeframeworkthatcanelongateeectiveπ-conjugationlengthandfacilitatethechargecarriermobility.
SinceKoetal.
developedtherstIDT-basedcopolymerwithaPCEof4.
4%[9],alargenumberofIDT-containingcopolymershavebeensynthesizedbythecopolymerizationbetweenvariousacceptorunitsanddierentIDTderivatives[10].
OnethingdeservestobementionedisthattheIDTcanbealsousedfortheconstructionofn-typesemiconductingmaterialsasecientnon-fullereneacceptors[11].
ConsideringthefactthatbothBTOandIDTarebulkyaromaticunitswithlongalkylchains,asuitableπ-bridgemodulationmaygreatlyaecttheperformanceofthecopolymersbasedonthem.
Itisexpectedthatcopolymerswithamorelinearbackbonestructureandalargerspacebetweenthelongalkylchainsmayshowbetterphotovoltaicperformance.
Moreimportantly,theintroductionofπ-bridgeintotheIDT-BTOpolymerbackbonemayleadtoD-Acopolymersexhibitingwidebandgaps[4,7b].
Inthiscontext,wereporttwonovelDAcopolymers(PIDTBTO-T,PIDTBTO-TT)basedonanIDTdonorunitandaBTOacceptorunitspacedwiththiopheneandTTπ-bridges,respectively(Scheme1).
Theeectsoftheπ-bridgeonthepolymericcurvature,π-πpacking,optical,electrochemical,nano-sizedphaseseparation,carriertransportandphotovoltaicpropertiesofthetargetcopolymersareinvestigatedindetail.
ConventionalPSCsbasedonthetwocopolymersasthedonorsand[6,6]-phenylC71-butyricacidmethylester(PC71BM)astheacceptorhavebeenfabricated.
Asexpected,thecopolymerwithTTspacers(PIDTBTO-TT)showsasuperiorPCEupto7.
08%,whilePIDTBTO-TexhibitsamoderatePCEof6.
40%.
Afteraddingasmallamountof1,8-diiodooctane(DIO)asaprocessingadditive,thePIDTBTO-TT-baseddeviceexhibitsanincreasedPCEof8.
15%.
Forthersttime,ahybridelectrontransportlayerbasedonpoly[(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-uorene)-alt-2,7-(9,9-dioctyl-uorene)](PFN,Fig.
S1,SI)[2e]and2,9-bis(3-(dimethylamino)propyl)anthrax[2,1,9-def:6,5,10-d′e′f′]diisoquinoline-1,3,8,10(2H,9H)tetraone(PDIN,Fig.
S1,SI)[5a]isusedtofabricateatandemPSCwithPIDTBTO-TT:PC71BMasthebottomcellcomponent.
Thebestperfor-mancetandemdeviceexhibitsahighPCEof11.
15%withalargeVocof1.
70V,aJscof9.
68mAcm2,andadecentFFof67.
79%.
TheobtainedPCEsof8.
15%and11.
15%arethehighestvaluesreportedtodateforsingle-junctionandtandemcellsusingIDT-basedwidebandgapcopolymers,respectively.
2.
Resultsanddiscussion2.
1.
PolymersynthesisandcharacterizationThecopolymersPIDTBTO-TandPIDTBTO-TTwerepreparedviaStillecross-couplingpolymerizationbetweenthecorrespondingmono-mers(Scheme1)intolueneusingPd2(dba)3/P(o-tolyl)3orPd(PPh3)4catalyticsystem.
DuetotherelativelyhigherreactivityoftheBTO-TTmonomerincomparisonwiththeBTO-Tmonomer(Scheme1),alessreactivecatalyst,i.
e.
Pd(PPh3)4,wasusedforthesynthesisofPIDTBTO-TT,andamorereactivecatalyst,i.
e.
Pd2(dba)3/P(o-tolyl)3,wasusedforthesynthesisofPIDTBTO-T.
Furthermore,dierentcopolymerizationtimeswereusedtocontrolthemolecularweightsandtoensuregoodsolubilityofthetargetcopolymers.
Thenalcopolymersforthedevicefabricationwerefromthebatcheswiththehighestmolecularweights,andwithgoodsolubilityinchloroform,chlorobenzene,ando-dichlorobenzene(DCB).
Beforequenchingthepolymerizations,2-tributylstannylthiopheneand2-bromothiophenewereaddedsequentiallytoend-capthepolymerchainswiththiophene.
Thispreventsthereactiveendgroupsfromactingaschargetrappingsitesindevices.
Thecrudecopolymerswereprecipitatedinmethanol,andpuriedbySoxhletextractionwithmethanol,acetone,hexaneanddichloromethaneinsequencetoremoveanyresidualcatalystandlowmolecularweightoligomers.
Thenumberaveragemolecularweights(Mns)andpolydispersityindexes(PDIs)ofpolymersweremeasuredbygelpermeationchro-matography(GPC)using1,2,4-trichlorobenzeneastheeluentat150°C.
TheMnsofPIDTBTO-TandPIDTBTO-TTare97.
5and119.
9kDa,withPDIsof2.
5and1.
8,respectively(Table1).
Withsixlongalkylchainsoneveryrepeatunit,thesehighmolecularweightcopolymersstillhavegoodsolubilityincommonsolventssuchaschloroform,chlorobenzeneetc.
Thehighmolecularweightisdesirablebecauseitwillhelptoimprovethelm-formingabilityandtheresultingphotovoltaicperformance.
Thethermalstabilityofthecopolymerswasinvestigatedbythermogravimetricanalysis(TGA)(Fig.
S2,SI).
AsshowninFig.
S2,thetwocopolymershavegoodthermalstabilitywithdecompositiontemperatures(5%weightloss)over300°Cunderanitrogenatmosphere.
X-raydiraction(XRD)(Fig.
S3,SI)doesnotshowcleardiractionpeaksintherangeof2θ=0–20°,whichindicatesthatbothtwocopolymershavesimilaramorphouscharacteristicsasotherIDT-basedpolymers[9,10].
2.
2.
OpticalandelectrochemicalpropertiesTheUV–VisabsorptionspectraofPIDTBTO-TandPIDTBTO-TTinchloroformsolution(1*105M)andthinlmareshowninFig.
1andthedetailedopticalparametersaresummarizedinTable1.
Inbothsolutionandthinlm,PIDTBTO-TandPIDTBTO-TTexhibitverysimilarabsorptioncharacteristicsduetotheirapproximatepolymerbackbones.
Thehigherandlowerenergybands(300–500and500–700nm,respectively)areattributedtolocalizedπ-π*transitionsandintramolecularD-Achargetransfer,respectively.
PIDTBTO-TTexhibitsred-shiftedabsorptioninsolutioncomparedtoPIDTBTO-T,withtheabsorptionpeakgoingfrom574to585nm.
ThiscouldbeexplainedbythefactthatthehigherlinearityandextendedlengthoftheTTπ-bridgeY.
Maetal.
NanoEnergy33(2017)313–324314mayincreasetheplanarityofthepolymerbackboneandextendtheeectiveπ-conjugationlengthwhichissupportedbytheoreticalstudiesinthecomingsection.
Ingoingfromsolutiontosolidlm(Fig.
1b),negligiblechangesareobservedfortheabsorptionproles,exceptthatthereare18nmred-shiftedabsorptionpeaksforthethinlms.
Thisphenomenoncanbeassignedtothehigherstructuralorganizationandmoreorderedpackinginducedbythestrongerintermolecularinterac-tioninthesolidstate.
Theopticalbandgaps(Egopt)deducedfromtheabsorptionedges(λedge)ofbothpolymerlmsarearound1.
87eV.
Inordertodeterminetheenergylevelsandelectrochemicalproper-tiesofthecopolymers,thecyclicvoltammetry(CV)experimentsofthecopolymersinthinlmswereconducted.
ThevoltammogramsareshowninFig.
1candthedetailedresultsaresummarizedinTable1.
Theonsetpositionsofthep-dopingprocessofPIDTBTO-TandPIDTBTO-TTareat0.
57Vand0.
59VversusAg/Ag+,correspondingtothehighestoccupiedmolecularorbital(HOMO)levels(i.
e.
,ioniza-tionpotentials)of5.
39and5.
41eV,respectively.
Thelowestunoccupiedmolecularorbital(LUMO)levels(i.
e.
,electronanities)forPIDTBTO-TandPIDTBTO-TTare3.
48and3.
51eV,respec-tively,accordingtotheironsetreductionpotentials.
ThetrendofthesemeasuredenergylevelscoincideswellwiththecalculatedresultsbytheDFTcalculation(Fig.
S4,SI).
TherelativelydeepHOMOenergylevelScheme1.
SynthesisoftargetIDT-basedwidebandgapcopolymers.
Table1Summaryofthemolecularweights,optical,andelectrochemicalpropertiesofthecopolymers.
PolymerMnPDIλmax(nm)λmax(nm)EgoptHOMOLUMO(kDa)inCHCl3infilm(eV)a(eV)b(eV)cPIDTBTO-T97.
52.
55745931.
875.
393.
48PIDTBTO-TT119.
91.
85856021.
875.
413.
51aEstimatedfromtheonsetoftheabsorptionspectraofthinlms.
bEHOMO=(φox+4.
82)eV.
cELUMO=(φred+4.
82)(eV).
Fig.
1.
UV–visabsorptionspectraina)solutionandb)thinlm;c)cyclicvoltammogramsandd)energyleveldiagramofthecopolymers.
Y.
Maetal.
NanoEnergy33(2017)313–324315below5.
39eVofacopolymerisadvantageoustoobtainPSCswithhighVocs.
2.
3.
ConformationalanalysisbycalculationTogainmoreinsightintotheelectronicstructuresofthepolymerand,inparticulartheeectoftheπ-bridgeonthemolecularstructuresandelectronicproperties,computationalstudieswereperformedbydensityfunctionaltheory(DFT,B3LYP/6–311G**level)withachainlengthofn=2.
Hexylandoctylgroupsarereplacedbymethylgroupstosimplifythecalculation.
Forbothdimers,theHOMOwavefunctionsaredelocalizedalongthepolymerbackbone,whiletheLUMOwavefunctionsaremorelocalizedattheacceptorunits(Fig.
2).
Meanwhile,amoreextendeddelocalizationoftheHOMOwasobservedforPIDTBTO-TTcomparedtothatforPIDTBTO-T.
AsshowninFig.
2,thesetwocopolymerbackbonesshowsimilarlinearconformationintheirtopviews.
However,sideviewsshowthatPIDTBTO-Thasafairlycurvedbackbonewithatorsionalangleof~19°(TableS1,SI)betweenthetwoclosestIDTunitsalongthebackbone.
Onthecontrary,PIDTBTO-TTshowsahigherplanaritywithaverysmalltorsionalangleof~1.
6°,whichwouldfacilitateπ-electrondelocalizationandenhancechargecarriermobility.
Theseresultsimplythatthereplace-mentofthethiopheneπ-bridgewiththeTTunithasalargeeectonthegeometryofthepolymerbackbones.
2.
4.
FieldeecttransistorsToevaluatetheeectofstructuralmodicationofpolymersontheirholemobility,organiceld-eecttransistors(OFETs)basedonpristinePIDTBTO-TandPIDTBTO-TT,andwithabottom-gatetop-contactdevicecongurationwerefabricatedonahighlyn-dopedsiliconwafer.
TypicaloutputandtransfercurvesareshowninFig.
3,andtheparametersaresummarizedinTable2.
Ascanbeseenfromtheirtransfercurves,bothpolymersexhibittypicalp-typecharacter-istics.
ThecalculatedholemobilitiesforPIDTBTO-TandPIDTBTO-TTare(3.
3±0.
3)*103and(9.
1±0.
1)*103cm2V1s1,respectively.
Thedevicesbasedonbothtwocopolymersexhibitclearon/obehaviorwhichwillminimizeleakagecurrents.
TheholemobilityofPIDTBTO-TTismorethantwotimeshigherthanthatofPIDTBTO-Twhichisassociatedwiththegreaterbackboneplanarityfortheformer.
TheresultsconrmthattheinsertionoftheTTπ-bridgecanimprovethechargetransportpropertyofIDT-basedcopolymerswhichmayberelatedtotheimprovedπ-πstackinginthinlm.
Obviously,comparedtoPIDTBTO-T,PIDTBTO-TThassomeadvantagesinphotovoltaicapplicationswithitshigherholemobility,althoughtheseOFETmobilitiesonlyrepresentthechargetransportpropertyofpurepolymerlminthelateraldirectionofsubstrate.
Grazingincidentwide-angleX-ray(GIWAXS)[12]isfurtheremployedtoinvestigatethemolecularpackingandcrystallinityofpurePIDTBTO-TandPIDTBTO-TTlms.
The2DGIWAXSpatternsofbothpolymersareshowninFig.
4a,bandcorresponding1Dline-cutsinthein-planeandout-of-planedirectionsareshowninFig.
4c.
BothPIDTBTO-TandPIDTBTO-TT-basedlmsshowgentlelamellar(100)peaksinthein-planeandout-of-planedirectionsandnomoreotherlamellar(h00)peaksareobserved.
Thisindicatesthatbothpolymershaveweaklamellarmolecularpacking,whichcorrespondstotheamorphousnatureofthesecopolymers.
Thestrongout-of-plane(010)π-πstackingpeakslocatedat1.
441and1.
481(d-spacingsof4.
36and4.
25)forPIDTBTO-TandPIDTBTO-TT,respectively,suggestthatbothpolymershaveapreferentialface-onorientation.
The(010)peakintensityofPIDTBTO-TTishigherthanthatofPIDTBTO-T,andPIDTBTO-TThasacloserπ-πpackingdistancethanPIDTBTO-T.
Coherencelengthsof(010)peaksestimatedbyScherrerequation[13]are11.
5forPIDTBTO-Tand13.
1forPIDTBTO-TT,respectively.
Theimprovementofverticalπ-πstacking,whichisbenecialtochargetransportandreducingbimolecularrecombination,mainlycomesfromthebettermolecularplanarityofthePIDTBTO-TTthanPIDTBTO-Tasevidencedbythetheoreticalcalculation.
2.
5.
PhotovoltaicpropertiesofsinglejunctiondevicesThephotovoltaicpropertiesofbothtwocopolymerswereinvesti-Fig.
2.
DFT-calculatedLUMOandHOMOofthegeometryoptimizedstructuresofanalogousdimersofPIDTBTO-T(left),andPIDTBTO-TT(right).
Y.
Maetal.
NanoEnergy33(2017)313–324316gatedwithaconventionaldevicecongurationofITO/PEDOT:PSS/polymer:PC71BM/PDIN/Al.
Here,PDINwasemployedasthecathodeinterlayerbecauseitcanlowertheworkfunctionoftheelectrode,allowinghigherwork-functionmetals(suchasAl)toactasthecathode[5a].
PC71BMwaschosenastheelectronacceptorduetoitsbroaderandstrongerabsorptioninthevisibleregion,whichiscomplementarytotheabsorptionvalleyofthecopolymersandbenecialforhigheciencyPSCs[14].
Theactivelayerswerepreparedbyspincoatingthepolymer:PC71BMblendsonthetopofPEDOT:PSSlayerwithoutanyfurthertreatment.
ThemeasurementswereperformedundersimulatedAM1.
5G,100mW/cm2illuminationwithanactiveareaof0.
06cm2.
Thecurrentdensity-voltage(J-V)characteristicsofthesePSCsareshowninFig.
5a,andtheparametersofopencircuitvoltage(Voc),short-circuitcurrent(Jsc),llfactor(FF),andPCEaresummar-izedinTable3.
Initially,thedeviceperformancewasoptimizedthroughseveralaspectsasfollows:i)thepolymer:PC71BMblendratio,ii)theproces-singsolvent(CB:DCBratio),iii)theactivelayerthickness(TablesS2–S4,SI).
Undertheoptimaldevicepreparationconditions,thebestperformancedevicebasedonPIDTBTO-TshowsaPCEof6.
40%withVoc=0.
86V,Jsc=12.
36mAcm2,andFF=59.
90%.
Incontrast,thebestperformancedevicebasedonPIDTBTO-TTexhibitsanimprovedPCEof7.
08%withVoc=0.
88V,Jsc=13.
21mAcm2andFF=60.
85%.
TheVocofPIDTBTO-TT-baseddevicewashigherthanthatofPIDTBTO-T-basedcounterpart,whichcanbeattributedtothelower-lyingHOMOenergylevelofPIDTBTO-TT.
SincetheVocofagivenPSCisrelatedtotheenergyleveldierencebetweentheHOMOofthepolymerandtheLUMOofPC71BM.
TheincreasedJscandFFforthePIDTBTO-TT-baseddevicecouldbetheresultofoptimizedmorphologyandhigherholemobilityofthepolymerblend.
TheresultsdemonstratethatinsertingtheTTπ-bridgeintoIDT-BTO-basedpolymersystemisaneectivestrategytoenhanceJscandFFwithoutsacricingVocvaluesofPSCs.
Inordertofurtheroptimizethephotovoltaicperformanceofthetwocopolymersthroughlmmorphologytuning,1,8-diiodooctane(DIO)wasaddedintotheactivelayersolutionspriortothespin-coatingprocess(TableS5,SI).
When0.
7%(v/v)ofDIOwasadded,theVocandJscofthePIDTBTO-TT-baseddevicesincreaseto0.
91Vand14.
77mAcm2,respectively.
Asaconsequence,thebestperformancedevicebasedonPIDTBTO-TTexhibitsanincreasedPCEof8.
15%.
Tothebestofourknowledge,thiseciencyrepresentsthehighestvaluereportedsofarforsingle-junctionPSCsbasedonIDT-containingcopolymers.
Conversely,theadditionofDIO(0.
7%byvolume)tothePIDTBTO-T:PC71BMblendleadstoadrasticdropinPCE(4.
08%),withadecreaseinVoc(0.
84V),Jsc(10.
28mAcm2),andFF(47.
22%).
ThedropinVoccanbeattributedtotheloweredenergyofcharge-separatedstateupontheDIOaddition[15].
AndthedecreasesinJSCandFF,whenDIOwasused,couldberelatedtothemorphologychanges[16].
Toascertaintheaccuracyofthemeasurements,externalquantumeciencies(EQEs)ofthedeviceswithorwithoutDIOadditiveweremeasuredandshowninFig.
5b.
AlltheEQEspectracoverabroadspectralresponserangefrom300to700nm,whichagreewiththeabsorptionspectraofthepolymerblends(PIDTBTO-TT:PC71BMwasusedasanexample,Fig.
S5,SI).
Inthisrange,theaverageEQEvaluesofPIDTBTO-T-andPIDTBTO-TT-baseddeviceswithoutDIOare55%and58%,respectively.
AfteraddingDIO,theaverageEQEvalueschangeto45%and65%forPIDTBTO-T-andPIDTBTO-TT-baseddevices,respectively.
ThechangesinEQEareinagreementwiththecorrespondingchangesinJscaswellasPCEforbothcopolymers.
Atthesametime,theJscvaluescalculatedbyintegratingtheEQEdatawiththeAM1.
5GsolarspectrumareclosetothoseobtainedfromtheJ-Vmeasurementswithin3%mismatches.
Forexample,forthedeviceFig.
3.
TypicaloutputcharacteristicsofOFETsbasedonPIDTBTO-T(a)andPIDTBTO-TT(c);andtransfercharacteristicsofOFETsbasedonPIDTBTO-T(b)andPIDTBTO-TT(d).
Table2Mobilities,on-offcurrentratios,andthresholdvoltagesofOFETsbasedonthecopolymers.
Polymerμ(cm2V1s1)aIon/IobVt(V)cPIDTBTO-T(3.
3±0.
3)*103103(3–10)PIDTBTO-TT(9.
1±0.
1)*103103(4–10)aHolemobilitymeasuredatVD=80V.
bCurrentontooratio.
cThresholdvoltage.
Y.
Maetal.
NanoEnergy33(2017)313–324317basedonPIDTBTO-TTwithameasuredPCEof8.
15%,thecalculatedJscis14.
49mAcm2,onlyslightlylowerthanthemeasuredJscof14.
77mAcm2.
TodeterminetheoriginoftheobservedphotovoltaicperformancedierencesbetweenPIDTBTO-TTandPIDTBTO-Tandtheeectofthesolventadditive(DIO)ontheactivelayer,wemeasuredthechargetransportpropertiesoftheblendlmsbythespacechargelimitedcurrent(SCLC)method.
ItshouldbenotedherethattheOFETdataonlyrepresentthelateralmobilityatcarrierconcentrationgenerallygreaterthanthatinPSCs.
Toquantifythecarriermobilityperpendiculartothesubstrateplane,theSCLCmeasurementswerethereforecarriedout.
Thestructuresofhole-andelectron-onlydevicesareITO/PEDOT:PSS/polymer:PC71BM/MoO3/AuandITO/ZnO/polymer:PC71BM/Ca/Al,re-spectively.
TheholeandelectronmobilitiescalculatedfromtheSCLCmodelaresummarizedinTable3,andthecorrespondingJ–VcurvesunderdarkconditionareshowninFig.
S6(SI).
ForthedeviceswithoutDIO,theholemobilitiesare8.
1*106and1.
7*105cm2V1s1forPIDTBTO-TandPIDTBTO-TT,respectively;andtheelectronmobilitiesare2.
2*105and3.
1*105cm2V1s1forPIDTBTO-TandPIDTBTO-TT,respectively.
Thisexplainsthebetterphotovoltaicperformance,inparticularforthehigherJscandFFvaluesofthePIDTBTO-TT-basedPSCscomparedtothePIDTBTO-T-baseddevices.
AfteraddingDIOastheprocessingadditive,thePIDTBTO-TT:PC71BMlmshowsaslightlyincreasedholemobilityof2.
3*105cm2V1s1,andasimilarelectronmobilityof3.
5*105cm2V1s1.
Whereas,thePIDTBTO-T:PC71BMlmexhibitssignicantlydecreasedholeandelectronmobilitiesof1.
2*106and7.
6*106cm2V1s1,respectively.
ThisobservationisconsistentwiththecorrespondingPCEchangesinTable3.
Thehigherchargecarriermobilityandmorebalancedcarriertransport(reducedμe/μhratioinTable3)ofPIDTBTO-TT-baseddevicethanthoseofPIDTBTO-T-baseddeviceafteraddingDIOaremainreasonsfortheenhancedJscandFFofthecorrespondingdevice.
Tofurtherunderstandthephotovoltaicperformanceofthetwocopolymersaswellastheadditive(DIO)eectontheactivelayer,themorphologiesoftheactivelayerswereinvestigatedbytransmissionelectronmicroscopy(TEM)andatomicforcemicroscopy(AFM).
Fig.
6showstheTEMimagesoftheblendlms.
ItcanbefoundthatPIDTBTO-TandPIDTBTO-TTblendlmswithoutDIOshowsimilarphaseseparationwithdomainsizesof30–50nm.
Thus,theenhancedJscandFFofPIDTBTO-TT-baseddevicesmaybecontributedtothehighermobilityoftheactivelayer.
AftertheadditionofDIO,thelmmorphologiesofPIDTBTO-TandPIDTBTO-TTblendlmsexhibittwodistinctconversions.
InthecaseofthePIDTBTO-Tblend,theadditionofDIOleadstotheformationofapparentaggregateswithadomainsizeofmorethan100nm.
Thiskindofmorphologywillresultininecientexcitonseparationandchargetransport,andthusworsenthephotovoltaicperformanceofthecorrespondingdevice.
Incontrast,theadditionofDIOtothePIDTBTO-TTblendleadstoanimprovedmorphology.
TherelativelybigsizeaggregationsofpolymerandPC71BMdiminishedandabrousnetworkcanbeclearlyobserved.
Suchnetworkisbenecialforexcitonseparationandchargetransport,whichisingoodagreementwiththeaforementionedincreasedPCEandhole/electronmobility.
ThismorphologychangetrendscanalsobeobservedfromtheAFMimages(Fig.
S7,SI).
TheadditionofDIOtothePIDTBTO-Tblendlmleadstolargesizedomains,whicharenotfavourableforthetransportationofexcitontothedonor-acceptorinterface.
However,theadditionofDIOtothePIDTBTO-TTblendlmleadstomoreobviousphaseseparationwhichisbenecialforchargetransportation.
Atthesametime,blendlmsofPIDTBTO-TandPIDTBTO-TTwithPC71BMshowroot-mean-square(r.
m.
s.
)surfaceroughnessesof0.
23and0.
39nm,respectively.
AftertheadditionofDIO,theroughnessofthePIDTBTO-TT:PC71BMlmbarelychanges(0.
38nm),whereasthatforthePIDTBTO-T:PC71BMlmincreasesto2.
32nminagreementwiththeformationoflargeaggregates.
GIWAXSisalsousedtoinvestigatethemolecularpackingandcrystallinityofblendedlmswithandwithoutDIO.
The2DGIWAXSpatternsofthespin-coatedlmsareshowninFig.
S8a–d(SI)andcorresponding1Dline-cutsinthein-planeandout-of-planedirectionsareshowninFig.
S8e(SI).
Ingeneral,increasedout-of-plane(100)peakswithlongercoherencelengthsarefoundforthePIDTBTO-TTFig.
4.
2DGIWAXSpatternsofspin-coatedthinlmsofPIDTBTO-T(a)andPIDTBTO-TT(b),andthecorresponding1Dline-cutsinthein-planeandout-of-planedirections(c).
Y.
Maetal.
NanoEnergy33(2017)313–324318blendlmwithDIOorwithoutDIOincomparisonwiththePIDTBTO-Tblendlm,suggestingthebettercrystallinityoftheformercopoly-mer.
TheseresultsareinagreementwiththeimproveddeviceperformanceforPIDTBTO-TTincomparisonwithPIDTBTO-T.
However,the2DGIWAXSresultdoesnotrevealsignicantadditiveeectonthecrystallinityandthepackingdistanceforbothcopolymerblends.
2.
6.
PhotovoltaicpropertiesoftandemdevicesToinvestigatetheapplicationofthesewidebandgapcopolymersinmulti-junctionPSCs,double-junctiontandemcellswithadevicestructureofITO/PEDOT:PSS/PIDTBTO-TT:PC71BM/PDIN:PFN/Al/MoO3/PTB7-Th:PC71BM/PDIN/Al(Fig.
7a)werefabricatedasanexample.
ThechemicalstructuresofPTB7-Th,PDINandPFNareshowninFig.
S1(SI).
Here,thelowbandgappolymerPTB7-Thwasselectedasthetopcellactivematerialbecauseithasalowbandgapof1.
58eVwithastrongabsorptionbandintherangeof550–780nm(Fig.
7b)[2c–e],whichissomewhatcomplementarytotheabsorptionproleofPIDTBTO-TT.
AsshowninFig.
7a,PDINblendedwithPFNwas,forthersttime,usedastheelectrontransportlayer(ETL)forbottomsub-cell,andmolybdenumoxide(MoO3)wasemployedastheholetransportlayerforthetopsub-cell.
Meanwhile,anultra-thinAl(1nm)lmwasinsertedbetweenthePDIN:PFNandMoO3layerstoestablishanohmiccontact.
ThePDIN:PFNETLwouldhavecombinedadvantagesofthePDINandPFNlayers.
Forexample,thepoorlmformingpropertiesofPDINcanbeimprovedbyblendingwiththePFNcopolymer.
Atthesametime,thepresenceofPDINmayprotectPFNfrombeingdamagedbychlorinatedsolvents.
Moredetailedinvestiga-tiononthisblendedETLisstillunderwayandwillbepublishedinduecourse.
ThetandemdeviceswereoptimizedbyvaryingtheETLintheinterconnectinglayerandtheactivelayerthicknesses.
TheresultsshowthatthedevicesusingPDIN:PFNastheETLandtheactivelayerwiththicknessesof141and85nmforbottomandtopcellsgivethehighestPCEs(Figs.
S9–S10,TablesS6–S7,SI).
J-VcharacteristicsofthebestperformancetandemPSCsandcorrespondingsingle-junctionrefer-encesub-cellsareshowninFig.
7c,andthedetailedperformanceparametersaresummarizedinTable4.
ThebestperformancetandemcellshowsahighPCEof11.
15%withJsc=9.
68mAcm2,Voc=1.
70V,FF=67.
79%.
Withthesameactivelayers(thesamethicknessaswell),thereferencebottom(PIDTBTO-TT:PC71BM)andtop(PTB7-Th:PC71BM)sub-cellsshowmoderatePCEsof7.
34%and7.
86%,respectively.
TheircorrespondingEQEspectraareshowninFig.
S11(SI)withsmallspectralmismatches(720nm)andabandpasslter(380–420nm),respectively.
Thebeamsizeofthelightsourceisslightlysmallerthanthedeviceareatoavoiderrors.
4.
5.
OFETfabricationandcharacterizationTop-contact/bottom-gateOFETdeviceswerefabricatedusingSi/SiO2substrateswhereSiandSiO2wereusedasthegateelectrodeandgatedielectric,respectively.
Thesubstratesweresubjectedtocleaningbyultrasonicationinpiranhasolution(H2SO4/H2O2=3/1),deionizedwater,acetone,andisopropanol,anddriedinoven.
Tooptimizedeviceperformance,thesubstratesweremodiedwithoctadecyltrimethox-ysilane(OTS)toformaself-assembledmonolayer.
Thinlmsofthepolymersweredepositedonthetreatedsubstratesbyspincoatingapolymersolution(6mg/mL)inDCBundernitrogen.
Afterpolymerthinlmdeposition,about40nmthickgoldelectrodewasdepositedassourceanddraincontactsusingashadowmask.
TheOFETdeviceshadachannellength(L)of300mandachannelwidth(W)of6mm.
TheevaluationsoftheOFETswerecarriedoutinambientatmosphereusinganAgilent4155Csemiconductorparameteranalyzeronaprobestation.
Thecarriermobility,μ,wascalculatedfromthedatainthesaturatedregionaccordingtotheequation:(Id)sat=(W/2L)μCi(Vg–Vth)2,where(Id)satisthedraincurrentinthesaturatedregion,WandLarethesemiconductorchannelwidthandlength,respectively,Ci(Ci=10nF)isthecapacitanceofthegatedielectriclayer,andVgandVtharethegatevoltageandthethresholdvoltage,respectively.
Thesaturationregionmobilitieswerecalculatedfromthetransferchar-acteristicsoftheFETsusingtheslopederivedfromthesquarerootoftheabsolutevalueofthecurrentasafunctionofgatevoltagebetween80and20V.
4.
6.
Hole-andelectron-onlydevicefabricationandcharacterizationHole-onlydiodeswerefabricatedonITOcoatedglasswithaPEDOT:PSSbottomcontactandaMoO3/Autopcontact.
Electron-onlydiodeswerefabricatedonITOcoatedglasswithaZnObottomcontactandaCa/Altopcontact.
TheBHJlmswerepreparedusingthesamemethodasthatusedintheoptimalsolarcellfabrication.
Deviceareaswere6mm2.
Thecurrentdensity(J)wasmeasuredbyaKeithley2440sourcemeasurementunit.
TheSCLCholemobilitieswerecalculatedaccordingtothefollowingequation:[20]JεεμVL=98r023whereε0isthepermittivityoffreespace(8.
85*1012Fm1),εristhedielectricconstantofthepolymer(assumedtobe3,whichisatypicalvalueforconjugatedpolymers),μisthecarriermobility,Visthevoltagedropacrossthedevice(V=VapplVbi,whereVapplistheappliedvoltagetothedevice,andVbiisthebuilt-involtageduetothedierenceinworkfunctionofthetwoelectrodes),andListhepolymerthickness.
ThethicknessofthelmwasmeasuredbyaBrukerDektakXTsurfaceprolometer.
4.
7.
GIWAXScharacterizationGIWAXSmeasurementswereperformedatbeamline7.
3.
3attheAdvancedLightSource(ALS).
SampleswerepreparedonSisubstratesusingidenticalpolymersolutionsasthoseusedinFETorOPVdevices.
The10keVX-raybeamwasincidentatagrazingangleof0.
11–0.
15°,whichmaximizedthescatteringintensityfromthesamples.
ThescatteredX-raysweredetectedusingaDectrisPilatus1Mphotoncountingdetector.
4.
8.
SynthesisofPIDTBTO-TIDT(0.
24g,0.
19mmol)andBTO-T(0.
14g,0.
19mmol)weredissolvedintoluene(20mL).
Themixturewaspurgedwithnitrogenfor1hatroomtemperature.
AftertheadditionofPd2(dba)3(4mg)andP(o-tolyl)3(10mg),themixturewasheatedtoreuxfor12hunderanitrogenatmosphere.
Then,adropof2-tributylstannylthiophenewasaddedtothemixtureandreactedfor3h.
Finally,twodropsof2-bromothiophenewasaddedtothemixtureandreactedovernighttocompletetheend-cappingreaction.
Thereactionmixturewasprecipi-tatedintomethanolandltered.
Thecollectedprecipitatewassub-jectedtoSoxhletextractionwithmethanol,acetone,hexane,andchloroformfor24heach.
Thechloroformextractwasconcentrated,andthenprecipitatedintomethanol.
Thetargetpolymerwascollectedbyltrationanddriedinvacuoat50°Covernighttogiveablacksolid(0.
25g,89%).
1HNMR(CDCl3,400MHz,ppm):8.
49(br,2H),7.
45(br,2H),7.
30(br,4H),7.
24(br,8H),7.
14(br,8H),4.
17(br,4H),2.
61(br,8H),1.
98(br,4H),1.
64(m,8H),1.
51(m,4H),1.
34(m,40H),0.
90(m,18H).
GPC:Mn=97.
5kDa,PDI=2.
5.
4.
9.
SynthesisofPIDTBTO-TTIDT(0.
31g,0.
25mmol)andBTO-TT(0.
21g,0.
25mmol)weredissolvedintoluene(20mL).
Themixturewaspurgedwithnitrogenfor1hatroomtemperature.
Aftertheadditionof14.
5mgofPd(PPh3)4,themixturewasheatedtoreuxfor9hunderanitrogenatmosphere.
Then,adropof2-tributylstannylthiophenewasaddedtothemixtureandreactedfor3h.
Finally,twodropsof2-bromothiophenewasaddedtothemixtureandreactedovernighttocompletetheend-cappingreaction.
Thereactionmixturewasprecipitatedintomethanolandltered.
ThecollectedprecipitatewassubjectedtoSoxhletextractionwithmethanol,acetone,hexane,dichloromethaneandchloroformforY.
Maetal.
NanoEnergy33(2017)313–32432224heach.
Thechloroformextractwasconcentratedandthenpre-cipitatedintomethanol.
Thetargetpolymerwascollectedbyltrationanddriedinvacuoat50°Covernighttogiveablacksolid(0.
28g,72%).
1HNMR(CDCl3,400MHz,ppm):8.
84(br,2H),7.
45(br,2H),7.
41(br,4H),7.
25-7.
13(m,16H),4.
18(br,4H),2.
61(br,8H),2.
03(br,4H),1.
59(m,8H),1.
51(m,4H),1.
33(m,4H),0.
91(m,18H).
GPC:Mn=119.
9kDa,PDI=1.
8.
AcknowledgementsThisworkwassupportedbytheNationalNaturalScienceFoundationofChina(Nos.
U1605241,61325026,51561165011),theStrategicPriorityResearchProgramoftheChineseAcademyofSciences(No.
XDB20000000),andtheCAS/SAFEAInternationalPartnershipProgramforCreativeResearchTeams.
TheGIWAXScharacterizationissupportedbytheDirector,OceofScience,OceofBasicEnergySciences,oftheU.
S.
DepartmentofEnergyunderContractNo.
DE-AC02-05CH11231.
AppendixA.
SupplementarymaterialSupplementarydataassociatedwiththisarticlecanbefoundintheonlineversionathttp://dx.
doi.
org/10.
1016/j.
nanoen.
2017.
01.
050.
References[1](a)J.
Chen,Y.
Cao,Acc.
Chem.
Res.
42(2009)1709–1718(b)G.
Li,R.
Zhu,Y.
Yang,Nat.
Photonics6(2012)153–161(c)Y.
Li,Acc.
Chem.
Res.
45(2012)723–733(d)L.
Ye,S.
Q.
Zhang,L.
J.
Huo,M.
J.
Zhang,J.
H.
Hou,Acc.
Chem.
Res.
47(2014)1595–1603(e)J.
-S.
Wu,S.
-W.
Cheng,Y.
-J.
Cheng,C.
-S.
Hsu,Chem.
Soc.
Rev.
44(2015)1113–1154(f)L.
Lu,T.
Zheng,Q.
Wu,A.
M.
Schneider,D.
Zhao,L.
Yu,Chem.
Rev.
115(2015)12666–12731(g)W.
Li,K.
H.
Hendriks,M.
M.
Wienk,R.
A.
J.
Janssen,Acc.
Chem.
Res.
49(2016)78–85.
[2](a)Y.
Liu,J.
Zhao,Z.
Li,C.
Mu,W.
Ma,H.
Hu,K.
Jiang,H.
Lin,H.
Ade,H.
Yan,Nat.
Commun.
5(2014)5293(b)Y.
Liu,Z.
A.
Page,T.
P.
Russell,T.
Emrick,Angew.
Chem.
Int.
Ed.
54(2015)11485–11489(c)X.
Ouyang,R.
Peng,L.
Ai,X.
Zhang,Z.
Ge,Nat.
Photonics9(2015)520–524(d)J.
-D.
Chen,C.
Cui,Y.
-Q.
Li,L.
Zhou,Q.
-D.
Ou,C.
Li,Y.
Li,J.
-X.
Tang,Adv.
Mater.
27(2015)1035–1041(e)Z.
C.
He,B.
Xiao,F.
Liu,H.
B.
Wu,Y.
L.
Yang,S.
Xiao,C.
Wang,T.
P.
Russell,Y.
Cao,Nat.
Photonics9(2015)174–179(f)J.
B.
You,L.
T.
Dou,K.
Yoshimura,T.
Kato,K.
Ohya,T.
Moriarty,K.
Emery,C.
-C.
Chen,J.
Gao,G.
Li,Y.
Yang,Nat.
Commun.
4(2013)1446(g)N.
Li,C.
J.
Brabec,EnergyEnviron.
Sci.
8(2015)2902–2909(h)A.
R.
b.
M.
Yuso,D.
Kim,H.
P.
Kim,F.
K.
Shneider,W.
J.
daSilva,J.
Jang,EnergyEnviron.
Sci.
8(2015)303–316.
[3](a)F.
Guo,N.
Li,F.
W.
Fecher,N.
Gasparini,C.
O.
R.
Quiroz,C.
Bronnbauer,Y.
Hou,V.
V.
Radmilovic,V.
R.
Radmilovic,E.
Spiecker,K.
Forberich,C.
J.
Brabec,Nat.
Commun.
6(2015)7730(b)H.
Q.
Zhou,Y.
Zhang,C.
K.
Mai,S.
D.
Collins,G.
C.
Bazan,T.
Q.
Nguyen,A.
J.
Heeger,Adv.
Mater.
27(2015)1767–1773(c)K.
Zhang,K.
Gao,R.
Xia,Z.
Wu,C.
Sun,J.
Cao,L.
Qian,W.
Li,S.
Liu,F.
Huang,X.
Peng,L.
Ding,H.
-L.
Yip,Y.
Cao,Adv.
Mater.
28(2016)4817–4823(d)O.
Adebanjo,B.
Vaagensmith,Q.
Qiao,J.
Mater.
Chem.
A2(2014)10331–10349(e)N.
Li,D.
Baran,K.
Forberich,F.
Machui,T.
Ameri,M.
Turbiez,M.
Carrasco-Orozco,M.
Drees,A.
Facchetti,F.
C.
Krebs,C.
J.
Brabec,EnergyEnviron.
Sci.
6(2013)3407–3413(f)J.
Y.
Kim,K.
Lee,N.
E.
Coates,D.
Moses,T.
Q.
Nguyen,M.
Dante,A.
J.
Heeger,Science317(2007)222–225(g)C.
-Y.
Chang,L.
Zuo,H.
-L.
Yip,C.
-Z.
Li,Y.
Li,C.
-S.
Hsu,Y.
-J.
Cheng,H.
Chen,A.
K.
Y.
Jen,Adv.
EnergyMater.
4(2014)1301645(h)Z.
Zheng,S.
Zhang,J.
Zhang,Y.
Qin,W.
Li,R.
Yu,Z.
Wei,J.
Hou,Adv.
Mater.
28(2016)5133–5138(i)L.
Dou,J.
You,J.
Yang,C.
-C.
Chen,Y.
He,S.
Murase,T.
Moriarty,K.
Emery,G.
Li,Y.
Yang,Nat.
Photonics6(2012)180–185.
[4](a)Y.
Ma,Z.
Kang,Q.
Zheng,J.
Mater.
Chem.
A5(2017).
http://dx.
doi.
org/10.
1039/C6TA09325F(b)S.
C.
Price,A.
C.
Stuart,L.
Yang,H.
Zhou,W.
You,J.
Am.
Chem.
Soc.
133(2011)4625–4631(c)Y.
Dong,X.
W.
Hu,C.
H.
Duan,P.
Liu,S.
J.
Liu,L.
Y.
Lan,D.
C.
Chen,L.
Ying,S.
J.
Su,X.
Gong,F.
Huang,Y.
Cao,Adv.
Mater.
25(2013)3683–3688(d)C.
Cabanetos,A.
ElLabban,J.
A.
Bartelt,J.
D.
Douglas,W.
R.
Mateker,J.
M.
J.
Frechet,M.
D.
McGehee,P.
M.
Beaujuge,J.
Am.
Chem.
Soc.
135(2013)4656–4659(e)Y.
Ma,Q.
Zheng,L.
Wang,D.
Cai,C.
Tang,M.
Wang,Z.
Yin,S.
-C.
Chen,J.
Mater.
Chem.
A2(2014)13905–13915(f)J.
W.
Jung,F.
Liu,T.
P.
Russell,W.
H.
Jo,Adv.
EnergyMater.
5(2015)1503902(g)M.
Wang,D.
Cai,Z.
Yin,S.
-C.
Chen,C.
-F.
Du,Q.
Zheng,Adv.
Mater.
28(2016)3359–3365(f)M.
Wang,Z.
Wang,W.
Ma,S.
-C.
Chen,Q.
Zheng,Adv.
Electron.
Mater.
2(2016)1600340.
[5](a)Z.
-G.
Zhang,B.
Qi,Z.
Jin,D.
Chi,Z.
Qi,Y.
Li,J.
Wang,EnergyEnviron.
Sci.
7(2014)1966–1973(b)Z.
Yin,J.
Wei,Q.
Zheng,Adv.
Sci.
3(2016)1500362.
[6](a)X.
Wang,Y.
Sun,S.
Chen,X.
Guo,M.
Zhang,X.
Li,Y.
Li,H.
Wang,Macromolecules45(2012)1208–1216(b)X.
Guo,M.
J.
Zhang,L.
J.
Huo,F.
Xu,Y.
Wu,J.
H.
Hou,J.
Mater.
Chem.
22(2012)21024–21031(c)J.
J.
Intemann,K.
Yao,Y.
-X.
Li,H.
-L.
Yip,Y.
-X.
Xu,P.
-W.
Liang,C.
-C.
Chueh,F.
-Z.
Ding,X.
Yang,X.
Li,Y.
Chen,A.
K.
Y.
Jen,Adv.
Funct.
Mater.
24(2014)1465–1473(d)G.
Zuo,Z.
Li,M.
Zhang,X.
Guo,Y.
Wu,S.
Zhang,B.
Peng,W.
Wei,J.
Hou,Polym.
Chem.
5(2014)1976–1981.
[7](a)M.
H.
Chen,J.
Hou,Z.
Hong,G.
Yang,S.
Sista,L.
M.
Chen,Y.
Yang,Adv.
Mater.
21(2009)4238–4242(b)Q.
Zheng,B.
J.
Jung,J.
Sun,H.
E.
Katz,J.
Am.
Chem.
Soc.
132(2010)5394–5404(c)K.
Li,Z.
Li,K.
Feng,X.
Xu,L.
Wang,Q.
Peng,J.
Am.
Chem.
Soc.
135(2013)13549–13557.
[8](a)R.
P.
Qin,W.
W.
Li,C.
H.
Li,C.
Du,C.
Veit,H.
F.
Schleiermacher,M.
Andersson,Z.
S.
Bo,Z.
P.
Liu,O.
Inganas,U.
Wuerfel,F.
L.
Zhang,J.
Am.
Chem.
Soc.
131(2009)14612–14613(b)L.
Wang,D.
Cai,Z.
Yin,C.
Tang,S.
-C.
Chen,Q.
Zheng,Polym.
Chem.
5(2014)6847–6856(c)Y.
Ma,Q.
Zheng,Z.
Yin,D.
Cai,S.
-C.
Chen,C.
Tang,Macromolecules46(2013)4813–4821.
[9]C.
-P.
Chen,S.
-H.
Chan,T.
-C.
Chao,C.
Ting,B.
-T.
Ko,J.
Am.
Chem.
Soc.
130(2008)12828–12833.
[10](a)Y.
Zhang,J.
Zou,H.
-L.
Yip,K.
-S.
Chen,D.
F.
Zeigler,Y.
Sun,A.
K.
Y.
Jen,Chem.
Mater.
23(2011)2289–2291(b)M.
Zhang,X.
Guo,X.
Wang,H.
Wang,Y.
Li,Chem.
Mater.
23(2011)4264–4270(c)X.
Guo,M.
Zhang,J.
Tan,S.
Zhang,L.
Huo,W.
Hu,Y.
Li,J.
Hou,Adv.
Mater.
24(2012)6536–6541(d)H.
Bronstein,J.
M.
Frost,A.
Hadipour,Y.
Kim,C.
B.
Nielsen,R.
S.
Ashraf,B.
P.
Rand,S.
Watkins,I.
McCulloch,Chem.
Mater.
25(2013)277–285(e)M.
Wang,X.
Hu,L.
Liu,C.
Duan,P.
Liu,L.
Ying,F.
Huang,Y.
Cao,Macromolecules46(2013)3950–3958(f)D.
Liu,L.
Sun,Z.
Du,M.
Xiao,C.
Gu,T.
Wang,S.
Wen,M.
Sun,R.
Yang,RSCAdv.
4(2014)37934–37940(g)L.
Zuo,C.
-Y.
Chang,C.
-C.
Chueh,S.
Zhang,H.
Li,A.
K.
Y.
Jen,H.
Chen,EnergyEnviron.
Sci.
8(2015)1712–1718.
[11](a)Y.
Lin,J.
Wang,Z.
G.
Zhang,H.
Bai,Y.
Li,D.
Zhu,X.
Zhan,Adv.
Mater.
27(2015)1170–1174(b)Y.
Lin,Q.
He,F.
Zhao,L.
Huo,J.
Mai,X.
Lu,C.
-J.
Su,T.
Li,J.
Wang,J.
Zhu,Y.
Sun,C.
Wang,X.
Zhan,J.
Am.
Chem.
Soc.
138(2016)2973–2976.
[12](a)A.
A.
Y.
Guilbert,J.
M.
Frost,T.
Agostinelli,E.
Pires,S.
Lilliu,J.
E.
Macdonald,J.
Nelson,Chem.
Mater.
26(2014)1226–1233(b)A.
Hexemer,W.
Bras,J.
Glossinger,E.
Schaible,E.
Gann,R.
Kirian,A.
MacDowell,M.
Church,B.
Rude,H.
Padmore,in:Proceedingsofthe14thInternationalConferenceonSmall-AngleScattering(SAS09),Oxford,England,Sep13–18,2009.
[13](a)D.
-M.
Smilgies,J.
Appl.
Crystallogr.
46(2013)286(b)R.
Noriega,J.
Rivnay,K.
Vandewal,F.
P.
Koch,N.
Stingelin,P.
Smith,M.
F.
Toney,A.
Salleo,Nat.
Mater.
12(2013)1038–1044.
[14]M.
M.
Wienk,J.
M.
Kroon,W.
J.
H.
Verhees,J.
Knol,J.
C.
Hummelen,P.
A.
vanHal,R.
A.
J.
Janssen,Angew.
Chem.
Int.
Ed.
115(2003)3493–3497.
[15]D.
D.
Nuzzo,A.
Aguirre,M.
Shahid,V.
S.
Gevaerts,S.
C.
Meskers,R.
A.
Janssen,Adv.
Mater.
22(2010)4321–4324.
[16](a)C.
B.
Nielsen,R.
S.
Ashraf,N.
D.
Treat,B.
C.
Schroeder,J.
E.
Donaghey,A.
J.
P.
White,N.
Stingelin,I.
McCulloch,Adv.
Mater.
27(2015)948–953(b)H.
Zhong,Z.
Li,F.
Deledalle,E.
C.
Fregoso,M.
Shahid,Z.
Fei,C.
B.
Nielsen,N.
Yaacobi-Gross,S.
Rossbauer,T.
D.
Anthopoulos,J.
R.
Durrant,M.
Heeney,J.
Am.
Chem.
Soc.
135(2013)2040–2043(c)N.
Wang,Z.
Chen,W.
Wei,Z.
Jiang,J.
Am.
Chem.
Soc.
135(2013)17060–17068.
[17](a)A.
Martinez-Otero,Q.
Liu,P.
Mantilla-Perez,M.
M.
Bajo,J.
Martorell,J.
Mater.
Chem.
A3(2015)10681–10686(b)Y.
Liu,C.
-C.
Chen,Z.
Hong,J.
Gao,Y.
Yang,H.
Zhou,L.
Dou,G.
Li,Sci.
Rep.
3(2013)3356.
[18]J.
-H.
Kim,J.
B.
Park,F.
Xu,D.
Kim,J.
Kwak,A.
C.
Grimsdale,D.
-H.
Hwang,EnergyEnviron.
Sci.
7(2014)4118–4131.
[19]S.
Zeng,L.
Yin,C.
Ji,X.
Jiang,K.
Li,Y.
Li,Y.
Wang,Chem.
Commun.
48(2012)10627–10629.
[20]G.
G.
Malliaras,J.
R.
Salem,P.
J.
Brock,C.
Scott,Phys.
Rev.
B58(1998)13411–13414.
Y.
Maetal.
NanoEnergy33(2017)313–324323YunlongMareceivedhisB.
S.
degreefromMinjiangUniversityin2009,andM.
S.
degreeinmaterialsscienceandengineeringfromFujianNormalUniversityin2012.
HeiscurrentlypursuinghisPh.
D.
underthesupervisionofProf.
QingdongZheng.
Hisresearchinterestsincludethesynthesisoforganicsemiconductingmaterialsforpolymersolarcells.
Shan-CiChenreceivedhisB.
S.
degreeinchemistryfromXiamenUniversityin2005andaPh.
D.
degreeinphysicalchemistryfromFujianInstituteofResearchontheStructureofMatter,ChineseAcademyofSciencein2010.
HeisnowanassociateprofessorinProf.
QingdongZheng'sgroupattheFujianInstituteofResearchontheStructureofMatter,ChineseAcademyofSciences.
Dr.
Chen'sinterestinvolvesdesign,synthesis,andcharacterizationsofmateri-alsforvariousapplicationsincludingorganiceld-eecttransistors,organicsolarcellsetc.
ZaiyuWangreceivedhisB.
EnginMaterialsScience&EngineeringfromXi'anJiaotongUniversityin2015andjoinedProf.
Ma'sgroupthesameyear.
HeiscurrentlyworkingonthemorphologycharacterizationoforganicsolarcellsatXi'anJiaotongUniversityasagraduatestudent.
WeiMaobtainedhisPh.
D.
inChemicalPhysicsfromtheUniversityofPierreMarieCurie(Paris6,France)in2010beforemovingtotheEcoleNormaleSuperieure(Paris),France(2010–1011)andNorthCarolinaStateUniversity(NCSU)asPostdocFellow.
HejoinedtheSchoolofMaterialsScienceEngineeringatXi'anJiaotongUniversityin2014asaProfessor.
Hisresearchinterestsincludetheuseofsoft/hardX-raybasedtechniquestounraveltherelationshipbetweenlmmicrostructureanddeviceperformance.
JinyunWangreceivedherB.
S.
degreeineducationofchemistryfromFujianNormalUniversity,Chinain2006.
AndshegottheM.
S.
andPh.
DdegreesintheoreticalandcomputationalchemistryfromFujianInstituteofResearchontheStructureofMatter(FJIRSM),ChineseAcademyofSciencesin2008and2011,respectively.
Afterthat,sheservicedasanAssistantProfessorintheStateKeyLaboratoryofStructuralChemistryandChemistryDivisionatFJIRSM.
Herresearchprojectisrelatedtothetheoreticalstudyofthephotophysicalandelectron-trans-portpropertiesofthemetallorganiccomplexes.
ZhigangYinreceivedhisbachelordegreefromXi'anTechnologicalUniversityin2008andhismaster'sdegreeinmaterialsphysicsandchemistryfromFuzhouUniversityin2011.
HethenjoinedtheFujianInstituteofResearchontheStructureofMatter,ChineseAcademyofSciences(CAS),andbecameanassociateprofessorin2016.
Hisresearchinterestsincludeoptoelectronicmaterials,energydevices,andinterfacialsciences.
ChangquanTangreceivedhisB.
S.
degreefromJiangxiNormalUniversityin2007andM.
S.
degreeinorganicchemistryfromCentralSouthUniversityinChinain2010.
HeiscurrentlypursuinghisPh.
D.
intheUniversityofChineseAcademyofSciences.
Hisresearchinterestsfocusonthesynthesisandapplicationsofphoton-activemateri-als.
DongdongCaireceivedhisbachelordegreeandMaster'sdegreefromFujianNormalUniversityin2009and2012,respectively.
HethenjoinedtheFujianInstituteofResearchonthestructureofMatter,ChineseAcademyofSciences.
Hisresearchinterestsincludeorganicsemicon-ductormaterials,organicsolarcells,andorganiceldeecttransistors.
QingdongZhengreceivedhisB.
S.
degreeinnechemi-calsandhisM.
S.
degreeinappliedchemistryfromEastChinaUniversityofScienceandTechnology,Chinain1998and2001,respectively.
HeobtainedhisPh.
D.
degreeinchemistryfromtheStateUniversityofNewYorkatBualo,USAin2005.
AftercarryingouthispostdoctoralresearchattheJohnsHopkinsUniversity,hejoinedtheFujianInstituteofResearchontheStructureofMatter,ChineseAcademyofSciences,andbecameaprofessorin2010.
Hismaininterestsfocusonmultifunctionalmolecularmateri-alsanddevicesandinparticularontheeldsofsemi-conductingmaterialsandrelatedinterfacialmaterialsfororganicsolarcells.
Hehaspublishedmorethan80peer-reviewedpapers.
HeisservingasAssociateeditorofRSCAdvancesandastopicaleditorofChineseOpticsLetters.
Y.
Maetal.
NanoEnergy33(2017)313–324324
- atmosphereios11相关文档
- 支付ios11.0.2
- defaultios11.0.2
- eSc1Tios11.0.2
- 考试ios11.0.2
- 点击ios11.0.2
- 飞行器ios11.0.2
NameSilo域名优惠码活动
NameSilo是通过之前的感恩节优惠活动中认识到这家注册商的,于是今天早上花了点时间专门了解了NameSilo优惠码和商家的详细信息。该商家只销售域名,他们家的域名销售价格还是中规中矩的,没有像godaddy域名标价和使用优惠之后的价格悬殊很大,而且其特色就是该域名平台提供免费的域名停放、免费隐私保护等功能。namesilo新注册域名价格列表,NameSilo官方网站:www.namesilo....
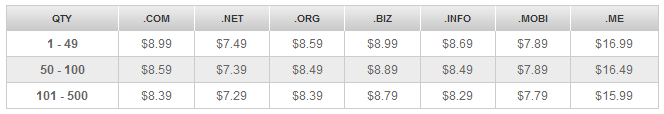
NameCheap域名转入优惠再次来袭 搜罗今年到期域名续费
在上个月的时候也有记录到 NameCheap 域名注册商有发布域名转入促销活动的,那时候我也有帮助自己和公司的客户通过域名转入到NC服务商这样可以实现省钱续费的目的。上个月续费转入的时候是选择9月和10月份到期的域名,这不还有几个域名年底到期的,正好看到NameCheap商家再次发布转入优惠,所以打算把剩下的还有几个看看一并转入进来。活动截止到9月20日,如果我们需要转入域名的话可以准备起来。 N...
青果云(59元/月)香港多线BGP云服务器 1核 1G
青果云香港CN2_GIA主机测评青果云香港多线BGP网络,接入电信CN2 GIA等优质链路,测试IP:45.251.136.1青果网络QG.NET是一家高效多云管理服务商,拥有工信部颁发的全网云计算/CDN/IDC/ISP/IP-VPN等多项资质,是CNNIC/APNIC联盟的成员之一。青果云香港CN2_GIA主机性能分享下面和大家分享下。官方网站:点击进入CPU内存系统盘数据盘宽带ip价格购买地...

ios11.0.2为你推荐
-
南京morphvox支付apple清华大学经济管理学院支持ipad支持ipad支持ipad司机苹果5重庆宽带测速重庆云阳电信宽带测速网址谁知道,帮个忙?windows键是哪个Win键是什么?x-routerX-TRAlL是什么意思