cm003hh.com
003hh.com 时间:2021-04-07 阅读:()
Determinationofmercurylevelsinenvironmentalsamplesusing2-acetylfuranisonicotinoylhydrazonebyspectrophotometricmethodFullPaperINTRODUCTIONTheanalyticalmonitoringofmercuryinenvironmen-tal,biological,industrialandfoodsamplesisextremelyimportantbecauseofthehightoxicityofthismetalbothinitsinorganicandorganiccompounds[1].
Anexampleforacutemercurypoisoningis"Mina-matadiseases"whichcausesmentaldisturbance,alossofbalance,speech,sightandhearing,difficultyinswallowingandfinallycomaanddeath[2].
Thetoxicityofmercuryde-pendsonitschemicalnature.
Inorganicmercuryhasaveryhighaffinityforproteinsulf-hydrylgroups,accu-mulatesinkidneys,whereasorganicmercuryismoreV.
SaleemBasha1,S.
VidyasagarBabu2,G.
Narasimha3,K.
HussainReddy2*1GovernmentDegreeCollege(Men),Anantapur,A.
P,(INDIA)2DepartmentofChemistry,SriKrishnadevarayaUniversity,Anantapur–515003.
A.
P,(INDIA)3AppliedMicrobiologylaboratory,DepartmentofVirology,SriVenkateswaraUniversity,Tirupati-517502AndhraPradesh,(INDIA)E-mail:khussainreddy@yahoo.
co.
in.
comtoxicsinceitissolubleinfat,thelipidfractionsofmem-branes,andbraintissue.
Mercurytoxicityiscausedmainlybythefactthatitentersthelivingorganismandreactswithdifferentenzymesinhibitingthecatalysisofbasicmetabolicreactions[3].
Theabilityoflivingorgan-ismstoconvertinorganicmercurytoorganicmercurycompoundswhicharemoretoxicandaccumulatetoagreaterextentinsensitivetissues,isalsoaconsiderablefact.
Themainsourcesofmercuryfromwhichitaccu-mulatesintheenvironmentaresyntheticadhesives,airconditionerfilters,amalgams,autoexhausts,anatomicpreservatives,antifoulingpaints,dentalamalgams,bat-teries,cathodetubes,bloodbanksaline,cinnabaretc.
KEYWORDSBiological;Soil;Environmentalsamples;Mercury(II);Substitutedhydrazones;Spectrophotometry.
ABSTRACT2-acetylfuranisonicotinoylhydrazone(AFINH)hasbeensynthesizedandproposedasanewchromogenicreagentforarapid,simple,selective,directandnon-extractivespectrophotometricmethodisdevelopedforthedeterminationofmercury(II)inaqueousdimethylformamide(DMF).
Thereagentgivesdeepyellowcoloured,1:1(M:L)complexwithmercury(II)insodiumacetate-aceticacidbuffermediumofpH5.
5atmax365nm.
Thecolourreactionisinstantaneousandtheabsorbanceremainsconstantforabout12hrs.
Themolarabsorptivityofmercurycomplexis2.
3x103Lmol-1cm-1andsandell'ssensitivityarefoundtobe0.
0888gcm-2.
Themethodwassuccessfullyappliedtoanumberofenvironmental,biologicalandsoilsamples.
Theresultswerecomparablewiththoseobtainedbydithizonemethod.
Theproposedsystemproducedsatisfactoryresultsforthedeter-minationofHg(II).
Theresultsarequiteencouragingandcanbringaware-nessamongthepublic.
2013TradeScienceInc.
-INDIAACAIJ,13(3)2013[81-85]AnIndianJournalVolume13Issue3AnalyticalCHEMISTRYAnalyticalCHEMISTRYISSN:0974-7419.
82Determinationofmercurylevelsinenvironmentalsamplesusing2-acetylfuranFullPaperACAIJ,13(3)2013K.
HussainReddyetal.
83FullPaperACAIJ,13(3)2013AnIndianJournalAnalyticalCHEMISTRYAnalyticalCHEMISTRYDeterminationofmercury(II)Mercury(II)reactswithAFINHinacidicpH(5.
5)togivecolouredcomplexes.
Thecolourreactionisin-stantaneousevenatroomtemperature.
Theorderofadditionofreagent,metalion,buffer,2.
5mlofDMF.
Theabsorbanceofthecolouredcomplexremainscon-stantformorethan2hours.
A10-foldmolarexcessofthereagentisadequateforfullcolourdevelopment.
Ad-ditionofexcessofreagenthasnoadverseeffectontheabsorbanceofthecomplexes.
ThesystemobeysBeer'slawintheconcentrationrange3.
21-32.
1g/mlofmer-cury.
ThemolarabsorptivityandSandell'ssensitivityofthemethodsforHg(II)arefoundtobe0.
41x104Lmol-1cm-1and0.
0875g/cm2respectively.
Thespe-cificabsorptivityofthesystemis0.
01142ml/g-1cm-1Hg(II).
Therelativestandarddeviationfortenrepli-cateanalysisofHg(II)is0.
082percent.
Job'sandMolarratiomethodsgavethecompositionoftheHg(II)complexesas1:2(M:L).
thestabilityconstantsofHg(II)complexcalculatedbyJob'smethodisfoundtobe1.
5x1010.
Determinationofmercuryinvariouswater,soilandbiologicalsamplesThepresentmethodisappliedfordeterminationofmercuryin(i)Watersamples,(ii)Soilsamples,and(iii)Biologicalsamples.
Watersamples[4,9]Eachfiltered(withwhattmanNo.
40)watersample[4,9](250ml)wasmixedwith10mlofconcen-tratednitricacidina500mldistillationflask.
Thesamplewasdigestedinthepresenceofanexcesspotassiumpermanganatesolutionaccordingtothemethodrec-ommendedbyFifieldetal[5].
ThesolutionwascooledandneutralizedwithdiluteNH4OHsolution.
Thedigestwastransferredintoa25-mlcalibratedflaskanddi-luteduptothemarkwithdeionizedwater.
TheresultsweregiveninTABLE2.
Soilssamples[5,9]andBiologicalsamples[6,9]Driedfishsamples[6,9]andvarioussoil[5,9]samples2-5gramsweretakenina250mlbeaker.
A6mlofconcentratednitricacidwasaddedandgentlyheatedforhalf-an-hour.
Afterthedisappearanceofthefroth,6mlof1:1nitricacidandperchloricacidwereadded[8-9].
Thecontentsweredigestedfor1hourandrepeatedlytreatedwith6mlportionsofnitricacidandpercholoricacidmixtureuntilthesolutionbecomescolourless.
Theacidicsolutionwasevaporatedtodrynessandthere-sultingwhiteresiduewasdissolvedinminimumvolumeproton,-NH(imino)groupsofhydrazonerespectively.
MassspectrumofAFINHshowsmolecularionpeakatm/z252correspondingtothemolecularionpeakassociatedwithNa(Basepeak).
Otherpeaksduetolossofmethylradicalandfuranylradicalarealsoobservedinmassspectrum.
ThepKavaluesofAFINHThepKavaluesofAFINHweredeterminedbyrecordingtheUV-Visiblespectraofmicromolar(4x10-6M)solutionofthereagentatvariouspHvaluesandbytakingthearithmeticmeansofthevaluesob-tainedfromthemeasurementsatdifferentwavelengthsdeterminedspectrophotometricallyusingPhillipandMerrittmethod[7].
ThevaluesofdeprotonationofAFINHare3.
6(pK1)and6.
3(pK2)correspondingtotheformationofenolformandconjugatedmonoanionformrespectively.
RESULTSANDDISCUSSIONThereagent2-acetylfuranisonicotinoylhydrazoneiseasilypreparedunderrefluxconditions.
A0.
001MsolutionofAFINHisstableformorethantwohours.
Inbuffermedium(pH5.
5),theligandpresumablyex-istsinenolicformandcoordinatesthedivalentmetalionasmonoanion.
Thereagentgivesintensecolourreactiononlywithmercuryandshowmaximumabsor-banceat355nm.
Thereagent(AFINH)isconsideredaspotentialreagentforselectivespectrophotometricdeterminationofmercury(II).
XCCH3NNHCONX=OFigure1:StructureofAFINH.
84Determinationofmercurylevelsinenvironmentalsamplesusing2-acetylfuranFullPaperACAIJ,13(3)2013K.
HussainReddyetal.
85FullPaperACAIJ,13(3)2013AnIndianJournalAnalyticalCHEMISTRYAnalyticalCHEMISTRYThepresentmethod(AFINH)wasappliedforthedeterminationofHg(II)whenpresentaloneandpresentinwater,biologicalandsoilsamplesandresultswerecomparedwithdithizonemethod[10].
APPLICATIONSMercurywasestimatedinvariouswatersamples,biologicalandsoilsamplesbyemployingthepresentmethod.
TheresultsarepresentedinTABLE2CONCLUSIONSThepresentstudyclearlyindicatesthatthemer-curycontentcanbedeterminedinultratracelevelinwater,biologicalandsoilsamplesusingpresentmethod.
Allthesefindingscausegreatconcernregardingpublichealthdemandinganaccuratedeterminationofthismetalionanditmayprovideawarenessamongthepublic.
TABLE3:DeterminationofmercuryinsoilsamplesAmountofmercuryafound(g/ml)NameofthesamplesAFINHmethodDithizonemethodUrbansoil0.
980.
98Agriculturalsoil0.
450.
41Roadsidesoil1.
681.
62aAverageofthreedeterminations.
TABLE4:DeterminationofmercuryinbiologicalsamplesAmountofmercuryafound(g/mlofdriedliver)LiversamplesAINHHmethodDithizonemethodFishliver1.
280.
84Sheepliver0.
830.
86aAverageoffivedeterminationsACKNOWLEDGEMENTTheAuthorSaleemisgratefultoUGCSEROHyderabadForthefinancialassistance,AuthoralsothankDrB.
VSubbaReddyIICTHyderabadandDr.
A.
RathanPrasad,Scientists,forprovidingIR,NMRandMassspectraldata.
REFERENCES[1]M.
HumairaKhan,JamaluddineAhmed;AnalyticalScience,21,507-512(2005).
[2]A.
I.
Vogel;ATextBookofQuantitativeInorganicAnalysis,3rdEdition;ELBSandLongman,325(1975).
[3]G.
Pavlogeorgaters,V.
Kikilias;GlobaNest,Int.
J.
,4(2-3),107-125(2002).
[4]S.
Hamads,S.
Motomizu,K.
Toei;Analyst,113,945-948(1988).
[5]D.
D.
Ferrin,D.
Boyd;BuffersforpHandmetaloncontrol,ChapmanandHall,London,9,128(1974).
[6]F.
W.
Fifield,P.
J.
Haines,(Eds);EnvironmentalAna-lyticalChemistry,BlackwellScience,378(2000).
[7]M.
Kamburova;Talanta,40(5),719-723(1993).
[8]K.
HussainReddy;Ph.
D.
thesis,S.
K.
University,(1983).
[9]KazumiInagakietal.
;TheAnalyst,125,191-196(2000).
[10]F.
D.
Snell,C.
T.
Snell;Colorimetricmethodsofanaly-sis,3rdEdition,11,92(1949).
[11]Z.
Marczenko;Spectrophotometricdeterminationofelements,Wiley,NewYork,241,351,602(1976).
[12]K.
B.
Chandrasekhar,K.
HussainReddy;Indian.
J.
Ch-em.
Sect.
A.
,40,727(2001).
Anexampleforacutemercurypoisoningis"Mina-matadiseases"whichcausesmentaldisturbance,alossofbalance,speech,sightandhearing,difficultyinswallowingandfinallycomaanddeath[2].
Thetoxicityofmercuryde-pendsonitschemicalnature.
Inorganicmercuryhasaveryhighaffinityforproteinsulf-hydrylgroups,accu-mulatesinkidneys,whereasorganicmercuryismoreV.
SaleemBasha1,S.
VidyasagarBabu2,G.
Narasimha3,K.
HussainReddy2*1GovernmentDegreeCollege(Men),Anantapur,A.
P,(INDIA)2DepartmentofChemistry,SriKrishnadevarayaUniversity,Anantapur–515003.
A.
P,(INDIA)3AppliedMicrobiologylaboratory,DepartmentofVirology,SriVenkateswaraUniversity,Tirupati-517502AndhraPradesh,(INDIA)E-mail:khussainreddy@yahoo.
co.
in.
comtoxicsinceitissolubleinfat,thelipidfractionsofmem-branes,andbraintissue.
Mercurytoxicityiscausedmainlybythefactthatitentersthelivingorganismandreactswithdifferentenzymesinhibitingthecatalysisofbasicmetabolicreactions[3].
Theabilityoflivingorgan-ismstoconvertinorganicmercurytoorganicmercurycompoundswhicharemoretoxicandaccumulatetoagreaterextentinsensitivetissues,isalsoaconsiderablefact.
Themainsourcesofmercuryfromwhichitaccu-mulatesintheenvironmentaresyntheticadhesives,airconditionerfilters,amalgams,autoexhausts,anatomicpreservatives,antifoulingpaints,dentalamalgams,bat-teries,cathodetubes,bloodbanksaline,cinnabaretc.
KEYWORDSBiological;Soil;Environmentalsamples;Mercury(II);Substitutedhydrazones;Spectrophotometry.
ABSTRACT2-acetylfuranisonicotinoylhydrazone(AFINH)hasbeensynthesizedandproposedasanewchromogenicreagentforarapid,simple,selective,directandnon-extractivespectrophotometricmethodisdevelopedforthedeterminationofmercury(II)inaqueousdimethylformamide(DMF).
Thereagentgivesdeepyellowcoloured,1:1(M:L)complexwithmercury(II)insodiumacetate-aceticacidbuffermediumofpH5.
5atmax365nm.
Thecolourreactionisinstantaneousandtheabsorbanceremainsconstantforabout12hrs.
Themolarabsorptivityofmercurycomplexis2.
3x103Lmol-1cm-1andsandell'ssensitivityarefoundtobe0.
0888gcm-2.
Themethodwassuccessfullyappliedtoanumberofenvironmental,biologicalandsoilsamples.
Theresultswerecomparablewiththoseobtainedbydithizonemethod.
Theproposedsystemproducedsatisfactoryresultsforthedeter-minationofHg(II).
Theresultsarequiteencouragingandcanbringaware-nessamongthepublic.
2013TradeScienceInc.
-INDIAACAIJ,13(3)2013[81-85]AnIndianJournalVolume13Issue3AnalyticalCHEMISTRYAnalyticalCHEMISTRYISSN:0974-7419.
82Determinationofmercurylevelsinenvironmentalsamplesusing2-acetylfuranFullPaperACAIJ,13(3)2013K.
HussainReddyetal.
83FullPaperACAIJ,13(3)2013AnIndianJournalAnalyticalCHEMISTRYAnalyticalCHEMISTRYDeterminationofmercury(II)Mercury(II)reactswithAFINHinacidicpH(5.
5)togivecolouredcomplexes.
Thecolourreactionisin-stantaneousevenatroomtemperature.
Theorderofadditionofreagent,metalion,buffer,2.
5mlofDMF.
Theabsorbanceofthecolouredcomplexremainscon-stantformorethan2hours.
A10-foldmolarexcessofthereagentisadequateforfullcolourdevelopment.
Ad-ditionofexcessofreagenthasnoadverseeffectontheabsorbanceofthecomplexes.
ThesystemobeysBeer'slawintheconcentrationrange3.
21-32.
1g/mlofmer-cury.
ThemolarabsorptivityandSandell'ssensitivityofthemethodsforHg(II)arefoundtobe0.
41x104Lmol-1cm-1and0.
0875g/cm2respectively.
Thespe-cificabsorptivityofthesystemis0.
01142ml/g-1cm-1Hg(II).
Therelativestandarddeviationfortenrepli-cateanalysisofHg(II)is0.
082percent.
Job'sandMolarratiomethodsgavethecompositionoftheHg(II)complexesas1:2(M:L).
thestabilityconstantsofHg(II)complexcalculatedbyJob'smethodisfoundtobe1.
5x1010.
Determinationofmercuryinvariouswater,soilandbiologicalsamplesThepresentmethodisappliedfordeterminationofmercuryin(i)Watersamples,(ii)Soilsamples,and(iii)Biologicalsamples.
Watersamples[4,9]Eachfiltered(withwhattmanNo.
40)watersample[4,9](250ml)wasmixedwith10mlofconcen-tratednitricacidina500mldistillationflask.
Thesamplewasdigestedinthepresenceofanexcesspotassiumpermanganatesolutionaccordingtothemethodrec-ommendedbyFifieldetal[5].
ThesolutionwascooledandneutralizedwithdiluteNH4OHsolution.
Thedigestwastransferredintoa25-mlcalibratedflaskanddi-luteduptothemarkwithdeionizedwater.
TheresultsweregiveninTABLE2.
Soilssamples[5,9]andBiologicalsamples[6,9]Driedfishsamples[6,9]andvarioussoil[5,9]samples2-5gramsweretakenina250mlbeaker.
A6mlofconcentratednitricacidwasaddedandgentlyheatedforhalf-an-hour.
Afterthedisappearanceofthefroth,6mlof1:1nitricacidandperchloricacidwereadded[8-9].
Thecontentsweredigestedfor1hourandrepeatedlytreatedwith6mlportionsofnitricacidandpercholoricacidmixtureuntilthesolutionbecomescolourless.
Theacidicsolutionwasevaporatedtodrynessandthere-sultingwhiteresiduewasdissolvedinminimumvolumeproton,-NH(imino)groupsofhydrazonerespectively.
MassspectrumofAFINHshowsmolecularionpeakatm/z252correspondingtothemolecularionpeakassociatedwithNa(Basepeak).
Otherpeaksduetolossofmethylradicalandfuranylradicalarealsoobservedinmassspectrum.
ThepKavaluesofAFINHThepKavaluesofAFINHweredeterminedbyrecordingtheUV-Visiblespectraofmicromolar(4x10-6M)solutionofthereagentatvariouspHvaluesandbytakingthearithmeticmeansofthevaluesob-tainedfromthemeasurementsatdifferentwavelengthsdeterminedspectrophotometricallyusingPhillipandMerrittmethod[7].
ThevaluesofdeprotonationofAFINHare3.
6(pK1)and6.
3(pK2)correspondingtotheformationofenolformandconjugatedmonoanionformrespectively.
RESULTSANDDISCUSSIONThereagent2-acetylfuranisonicotinoylhydrazoneiseasilypreparedunderrefluxconditions.
A0.
001MsolutionofAFINHisstableformorethantwohours.
Inbuffermedium(pH5.
5),theligandpresumablyex-istsinenolicformandcoordinatesthedivalentmetalionasmonoanion.
Thereagentgivesintensecolourreactiononlywithmercuryandshowmaximumabsor-banceat355nm.
Thereagent(AFINH)isconsideredaspotentialreagentforselectivespectrophotometricdeterminationofmercury(II).
XCCH3NNHCONX=OFigure1:StructureofAFINH.
84Determinationofmercurylevelsinenvironmentalsamplesusing2-acetylfuranFullPaperACAIJ,13(3)2013K.
HussainReddyetal.
85FullPaperACAIJ,13(3)2013AnIndianJournalAnalyticalCHEMISTRYAnalyticalCHEMISTRYThepresentmethod(AFINH)wasappliedforthedeterminationofHg(II)whenpresentaloneandpresentinwater,biologicalandsoilsamplesandresultswerecomparedwithdithizonemethod[10].
APPLICATIONSMercurywasestimatedinvariouswatersamples,biologicalandsoilsamplesbyemployingthepresentmethod.
TheresultsarepresentedinTABLE2CONCLUSIONSThepresentstudyclearlyindicatesthatthemer-curycontentcanbedeterminedinultratracelevelinwater,biologicalandsoilsamplesusingpresentmethod.
Allthesefindingscausegreatconcernregardingpublichealthdemandinganaccuratedeterminationofthismetalionanditmayprovideawarenessamongthepublic.
TABLE3:DeterminationofmercuryinsoilsamplesAmountofmercuryafound(g/ml)NameofthesamplesAFINHmethodDithizonemethodUrbansoil0.
980.
98Agriculturalsoil0.
450.
41Roadsidesoil1.
681.
62aAverageofthreedeterminations.
TABLE4:DeterminationofmercuryinbiologicalsamplesAmountofmercuryafound(g/mlofdriedliver)LiversamplesAINHHmethodDithizonemethodFishliver1.
280.
84Sheepliver0.
830.
86aAverageoffivedeterminationsACKNOWLEDGEMENTTheAuthorSaleemisgratefultoUGCSEROHyderabadForthefinancialassistance,AuthoralsothankDrB.
VSubbaReddyIICTHyderabadandDr.
A.
RathanPrasad,Scientists,forprovidingIR,NMRandMassspectraldata.
REFERENCES[1]M.
HumairaKhan,JamaluddineAhmed;AnalyticalScience,21,507-512(2005).
[2]A.
I.
Vogel;ATextBookofQuantitativeInorganicAnalysis,3rdEdition;ELBSandLongman,325(1975).
[3]G.
Pavlogeorgaters,V.
Kikilias;GlobaNest,Int.
J.
,4(2-3),107-125(2002).
[4]S.
Hamads,S.
Motomizu,K.
Toei;Analyst,113,945-948(1988).
[5]D.
D.
Ferrin,D.
Boyd;BuffersforpHandmetaloncontrol,ChapmanandHall,London,9,128(1974).
[6]F.
W.
Fifield,P.
J.
Haines,(Eds);EnvironmentalAna-lyticalChemistry,BlackwellScience,378(2000).
[7]M.
Kamburova;Talanta,40(5),719-723(1993).
[8]K.
HussainReddy;Ph.
D.
thesis,S.
K.
University,(1983).
[9]KazumiInagakietal.
;TheAnalyst,125,191-196(2000).
[10]F.
D.
Snell,C.
T.
Snell;Colorimetricmethodsofanaly-sis,3rdEdition,11,92(1949).
[11]Z.
Marczenko;Spectrophotometricdeterminationofelements,Wiley,NewYork,241,351,602(1976).
[12]K.
B.
Chandrasekhar,K.
HussainReddy;Indian.
J.
Ch-em.
Sect.
A.
,40,727(2001).
- cm003hh.com相关文档
- 展品003hh.com
- implied003hh.com
- 寄存器003hh.com
- peace003hh.com
- wait003hh.com
- Flowchart003hh.com
SugarHosts糖果主机六折 云服务器五折
也有在上个月介绍到糖果主机商12周年的促销活动,我有看到不少的朋友还是选择他们家的香港虚拟主机和美国虚拟主机比较多,同时有一个网友有联系到推荐入门的个人网站主机,最后建议他选择糖果主机的迷你主机方案,适合单个站点的。这次商家又推出所谓的秋季活动促销,这里一并整理看看这个服务商在秋季活动中有哪些值得选择的主机方案,比如虚拟主机最低可以享受六折,云服务器可以享受五折优惠。 官网地址:糖果主机秋季活动促...

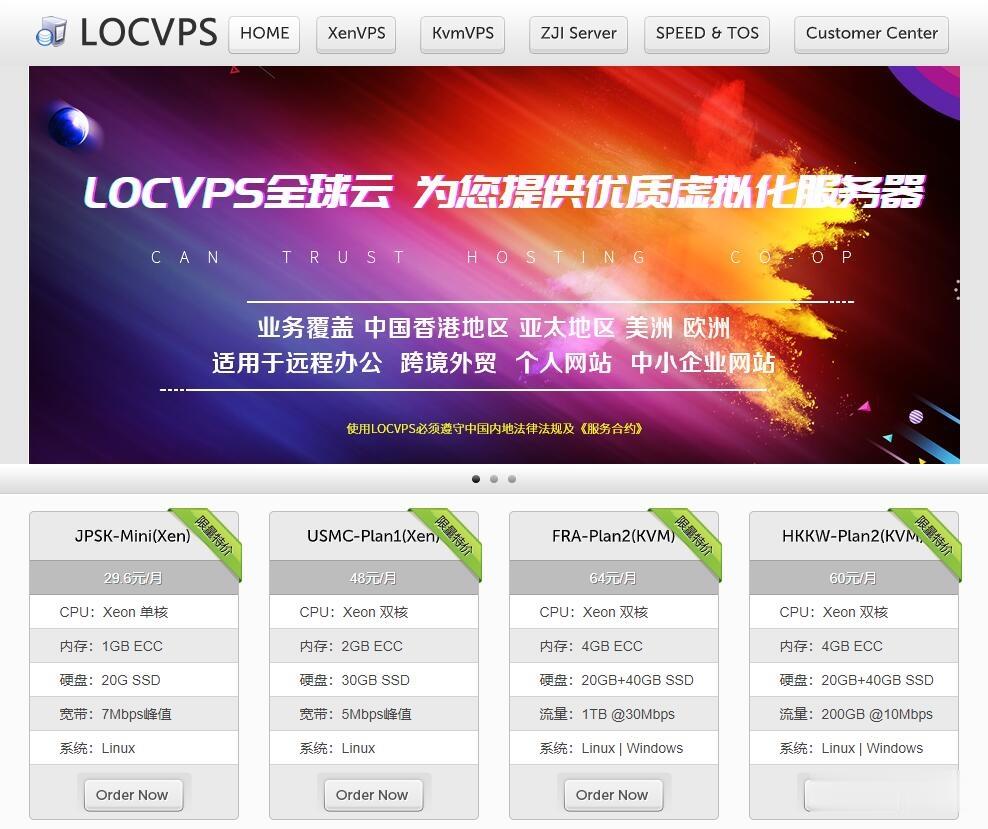
LOCVPS-2021年6月香港便宜vps宽带升级,充值就送代金券,其它八折优惠!
LOCVPS怎么样?LOCVPS是一家成立于2011年的稳定老牌国人商家,目前提供中国香港、韩国、美国、日本、新加坡、德国、荷兰等区域VPS服务器,所有机房Ping延迟低,国内速度优秀,非常适合建站和远程办公,所有机房Ping延迟低,国内速度优秀,非常适合做站。XEN架构产品的特点是小带宽无限流量、不超售!KVM架构是目前比较流行的虚拟化技术,大带宽,生态发展比较全面!所有大家可以根据自己业务需求...
牦牛云(3.5USD/月 )阿里云国际版云服务器 1核1G40G
收到好多消息,让我聊一下阿里云国际版本,作为一个阿里云死忠粉,之前用的服务器都是阿里云国内版的VPS主机,对于现在火热的阿里云国际版,这段时间了解了下,觉得还是有很多部分可以聊的,毕竟,实名制的服务器规则导致国际版无需实名这一特点被无限放大。以前也写过几篇综合性的阿里云国际版vps的分析,其中有一点得到很多人的认同,那句是阿里云不管国内版还是国际版的IO读写速度实在不敢恭维,相对意义上的,如果在这...

003hh.com为你推荐
-
微信回应封杀钉钉微信发过来的钉钉链接打不开?广东GDP破10万亿想知道广东城市的GDP排名www.yahoo.com.hk香港的常用网站www.kk4kk.com猪猪影院www.mlzz.com 最新电影收费吗?www.sesehu.comwww.121gao.com 是谁的网站啊www.zjs.com.cn怎么查询我的平安信用卡寄送情况百度指数词什么是百度指数www.5any.comwww.qbo5.com 这个网站要安装播放器ip查询器怎么样查看自己电脑上的IP地址javbibi日文里的bibi是什么意思