cryostagenano6
nano6 时间:2021-01-17 阅读:()
1Smallmoleculeicerecrystallizationinhibitorsmitigateredbloodcelllysisduringfreezing,transientwarmingandthawingJennieG.
Briard1,JessicaS.
Poisson1,TraceyR.
Turner2,ChantelleJ.
Capicciotti1,JasonP.
Acker2&RobertN.
Ben1Duringcryopreservation,icerecrystallizationisamajorcauseofcellulardamage.
Conventionalcryoprotectantssuchasdimethylsulfoxide(DMSO)andglycerolfunctionbyanumberofdifferentmechanismsbutdonotmitigateorcontrolicerecrystallizationatconcentrationsutilizedincryopreservationprocedures.
InNorthAmerica,cryopreservationofhumanredbloodcells(RBCs)utilizeshighconcentrationsofglycerol.
RBCunitsfrozenundertheseconditionsmustbesubjectedtoatime-consumingdeglycerolizationprocessafterthawinginordertoremovetheglycerolto0.
05)as4atonly5mMbutislessIRIactivethanboth3and4sug-gestingthatfactorsotherthanIRIactivitymaybeimportantforthecryoprotectiveactivity.
TheabilitytoreducetheamountofglycerolinthepresenceofanIRIwithoutcompromisingthenumberofRBCsrecoveredissignif-icantbecausecryopreservationusinglessglycerolwillreducepost-thawprocessingtimeintheclinicalsetting.
GiventhattheoptimalconcentrationsforthefreezingofRBCswerenotthesameasthoseutilizedfortheassessmentofIRIactivity(22mM),theIRIactivityof3–5wasre-assessedattheireffectiveinvitroconcentrationsreportedinFig.
4.
ThesedataarepresentedinFig.
5.
Asexpected,theIRIactivityof4doesnotchangedramati-cally,howevericecrystalsizeinthepresenceof110mM3isdramaticallysmallerinsize.
Interestingly,5appearedtobelesssensitivetoconcentrationeffectsasthereislittledifferenceinicecrystalsizedespitethefacttheconcen-trationisapproximatelyfour-foldless.
MeanIceCrystalSizeuponThawingFrozenRBCs.
AnalysisoficecrystalsizeisakeyaspectofthesplatIRIassaythatourlaboratoryhasdeveloped27.
PreviousworkfromourlaboratoryhasdemonstratedthataddingsmallmoleculeIRIsincreasespost-thawviabilityandallowsforareductionintheamountofcryopro-tectants4,10,16,28.
Wepredictedthaticecrystalsshouldbenoticeablysmallerinsizeinthepresenceofanicerecrys-tallizationinhibitor.
Thus,anexperimentwasperformedinwhichhumanRBCswerefrozenusingaLinkamCryostageandtheicewasimagedinthepresenceofcells.
Usingthisapproach,asolutionofRBCsin15%glycerolor15%glycerolwith4(30mM)wascooledatarateof25°C/mintoatemperatureof40°C.
Thesamplewasthenwarmedto10°Catarateof10°C/minandheldfor10minutespriortotakingapicture.
Figure6showstheFigure2.
Chemicalstructureofaryl-glycosides(3,4),aryl-aldonamide(5)andalysine-basednon-ionicsurfactant(6).
Figure3.
IRIactivityofsmallmolecules3–5at22mM.
IRIactivityisrepresentedasapercentmeangrainsize(%MGS)after30minutesofrecrystallizationat6.
4°Ccomparedtoaphosphatebufferedsaline(PBS)positivecontrolforicerecrystallization.
4imagesoficecrystalsina15%glycerolsolutionwithandwithoutRBCs.
Itisimportanttonotethatthepercentageoficeinthe15%glyceroland15%glycerolwith30mM4samplesstaysconstanteveninthepresenceofRBCs.
Inotherwords,thepresenceofRBCsdoesnotappeartoinfluencetheamountoficeinthesample.
However,lessiceisobservedinsamples(withandwithoutRBCs)when4(30mM)ispresentcomparedtothe15%glycerolcon-trol.
Ineachoftheseimages,thepercentageoffrozenfractionissmall.
Thisisbecausetheholdingtemperatureof10°Cisclosetothecolligativefreezingpointdepressionofthe15%glycerolsolution(4°C)andthereforealargefractionofthesampleisunfrozen29.
AstheIRIactivityof3–5wasassessedinconditionswithhighamountsoficepresent,theexperimentwasrepeatedatalowerholdingtemperaturetoensurehighericevolume.
Figure7showsimagesoficecrystalsizewhenRBCswerecooledto40°Catarateof25°C/minandthenwarmedatthesamerateto20°CandheldforFigure4.
OptimizationofIRI(3–5)concentrationforfreezingofhumanRBCsusing15%glycerol.
RBCswereincubatedfor10minuteswith15%glycerolor15%glycerolwithcompound3,4,or5atvariousconcentrations.
Sampleswereheldat5°Cforfiveminutesbeforecontrollednucleationusingforcepspre-cooledinliquidnitrogen.
Thiscontrollednucleationisperformedtoensurethaticenucleationoccursatthesamesub-zerotemperatureof5°Cineachvial.
Thesampleswereheldat5°Cforanadditionalfiveminutesbeforebeingcooledto40°C(1°C/min).
Uponstabilizationat40°C,thesampleswererapidlythawedina37°CwaterbathandthepercentageofintactRBCswasmeasured.
Thesefreezingconditionswererepeatedtwotosixteentimes(n=2–16)foreachfreezingsolution.
Errorbarsarereportedasthestandarderrorofthemean(SEM).
Asterisks(*)indicatesignificantdifferencedeterminedbyunpairedStudent'st-test(pNaNO6,328.
70;found,327.
95.
IceRecrystallizationInhibition(IRI)Activity.
SampleanalysisforIRIactivitywasperformedusingthe"splatcooling"methodaspreviouslydescribed30.
Allcarbohydratederivativesassessedweredissolvedinaphos-phatebufferedsaline(PBS)solutioncomprisedofsodiumchloride(8%w/v),disodiumphosphate(1.
44%w/v),potassiumchloride(0.
2%w/v)andmonopotassiumphosphate(0.
24%w/v)indistilledwateradjustedtopH7.
4withconcentrationhydrochloricacid.
A10μLdropletofthissolutionwasdroppedfromamicropipettethroughatwometerhighplastictube(10cmindiameter)ontoablockofpolishedaluminumpre-cooledtoapproximately80°C.
Thedropletfrozeinstantlyonthepolishedaluminumblockandwasapproximately1cmindiameterand20μmthick.
Thiswaferwasthencarefullyremovedfromthesurfaceoftheblockandtransferredtoacryostageheldat6.
4°Cforannealing.
ItisimportanttonotethatIRIassayshavetypicallyusedannealingtemperaturesrangingfrom4°Cto8°C.
Theannealingtemperatureof6.
4°Cwasutilizedbecausethisisthestandardinourlaboratory.
Afteraperiodof30minutesat6.
4°C,thewaferwasphotographedbetweencrossedpolarizingfiltersusingadigitalcamera(NikonCoolPix5000)fittedtothemicroscope.
Atotalofthreedropsforeachsamplewereassayedandthreeimagesweretakenfromeachwaferwiththeareaoftwelvecrystalsineachimagebeingquantified.
Imageanalysisoftheicewaferswasperformedusingadomainrecognitionsoftware(DRS)program10.
ThisprocessingemployedtheMicrosoftWindowsGraphicalUserInterfacetoallowausertovisuallydemarcateandstoretheverticesoficedomainsinadigitalmicrograph.
Thedatawasthenusedtocal-culatethedomainareas.
AlldatawasplottedandanalyzedusingMicrosoftExcel.
Themeangrain(oricecrystal)size(MGS)ofthesamplewascomparedtotheMGSofthecontrolPBSsolutionforthatsamedayoftesting.
IRIactivityisreportedasthepercentageoftheMGS(%MGS)relativetothePBScontrol.
Therefore,smallpercent-agesrepresentasmallMGS(smallicecrystals),whichisindicativeofhighIRIactivity.
Errorbarsarereportedasthestandarderrorofthemean(SEM).
BloodCollectionandPreparation.
AllRBCunitswereobtainedfromNetCAD(CanadianBloodServices'NetworkCentreforAppliedDevelopment).
Wholebloodwascollectedfromhealthyvolunteersusingstandard-izedphlebotomyguidelinesapprovedbyCanadianBloodServices(CBS).
Informedconsentwasobtainedfromalldonors.
AllexperimentalprotocolswereapprovedbyNetCADandCBS.
EthicsapprovalswereobtainedfromResearchEthicsBoard(REB)atCBSandtheUniversityofAlberta.
Forcryovialexperiments,wholebloodunitswerecollectedandprocessedbyNetCAD(Vancouver,BC).
Thewholebloodwasprocessedusingthebuffycoat(BC)methodtoproduceleukocytereducedSAGMRBCunits,whichhasbeenpreviouslydescribed31.
Forcry-omicroscopyexperiments,wholebloodwascollectedbystandardphlebotomytechniquesintoEDTAcollectiontubes,pooledintoa15mLconicaltubeandthenprocessedtoobtaintheRBCs.
Processingwasachievedbycen-trifugation(10min,4°C,2,200g)followedbyremovaloftheplasmaandBCfractions.
TheremainingRBCswerethenwashedtwicewith0.
9%saline/0.
2%dextrose(SD)followedbyresuspensionoftheRBCsinSDtoafinalhematocritof0.
50L/L.
ThepreparedRBCswereusedonthesamedayofpreparation.
RBCFreezingExperiments.
Thefreezingsolutionconsistsofa30%glycerolsolutionpreparedfromacommerciallyavailableglycerolsolution(57Glycerolyte,Baxter)bydilutingitwith0.
2%/0.
9%dextrose/saline(SD).
Anequalvolumeoffreezingsolutionwasaddedto150μLofRBCsforafinalvolumeof300μL.
Thefinalconcentrationsofallfreezingsolutionswereasindicatedintheresultsanddiscussion.
RBCsuspensionsweretransferredtocryotubesandincubatedatroomtemperaturefor10minutespriortoimmersioninamethanolbathcooledto5°C.
AthermocouplewasinsertedintoaRBC/15%glycerolsample(temperatureprobe)tomonitortemperatureat1secondintervals.
Oncetheinternalsolutionfromthetemperatureprobereached5°C,icenucleationwasinducedbytouchingtheoutsideoftheglasscryotubeswithpre-cooled(inliquidnitrogen)forceps.
Controllednucleationisperformedtoensurethaticenucleationoccursatthesamesub-zerotemperatureof5°Cineachvial.
RBCsampleswerethenheldat5°Cfor5minutes.
Sampleswerethencooledatarateof1°C/minto40°C,thenthawedimmediatelybyplungingina37°Cwaterbath.
Post-thawhematocrits(Hcts)andpercenthemolysiswasdeterminedforallfreezingexperimentsbycomparingthesupernatanthemoglobinconcentrationtototalhemoglobinconcentrationusingthecyanmethemoglobinDrabkin'smethod32.
Thesefreez-ingconditionswererepeatedtwotosixteentimes(n=2–16)foreachfreezingsolution.
PercentageofintactRBCswasgraphedinadditiontoerrorbarsreportedasthestandarderrorofthemean(SEM).
StatisticalsignificanceforalldatawasdeterminedbyunpairedStudent'st-testwitha95%confidencelevel.
TransientWarmingExperiments.
Thefreezingsolutionconsistsofa30%glycerolsolutionpreparedfromacommerciallyavailableglycerolsolution(57Glycerolyte,Baxter)bydilutingitwith0.
2%/0.
9%dextrose/saline(SD).
Anequalvolumeoffreezingsolutionwasaddedto150μLofRBCsforafinalvolumeof300μL.
Thefinalconcentrationsofallfreezingsolutionswereasindicatedintheresultsanddiscussion.
RBCsuspensionsweretransferredtocryotubesandincubatedatroomtemperaturefor10minutespriortoimmersionindryice(80°C).
Atemperatureprobewasusedfortemperaturemeasurementsat1secondintervals.
Oncetheinternalsolutionreached80±2°C,thesampleswereimmersedinamethanolbathcooledto20°C.
Oncetheinternalsolutionreached20°C,thesampleswereplungedintodryiceagain.
RBCsampleswereheldindryiceuntiltheinternalsolutionfromthetemperatureprobereached80±2°C,afterwhichthesampleswereeitherthawed(representingonecycleoftransientwarming)orimmersedinamethanolbathcooledto20°C.
One,threeandfivecyclesofimmersionina20°Cmethanolbathanddryicewereperformed.
Sampleswerethawedquicklybyplungingina37°Cwaterbath.
Post-thawHctsandpercenthemolysiswasdeterminedforallfreezingexper-imentsbycomparingthesupernatanthemoglobinconcentrationtototalhemoglobinconcentrationusingthe9cyanmethemoglobinDrabkin'smethod32.
Thesefreezingconditionswererepeatedtwotosixtimes(n=2–6)foreachfreezingsolution.
PercentageofintactRBCswasgraphedinadditiontoerrorbarsreportedasthestandarderrorofthemean(SEM).
StatisticalsignificanceforalldatawasdeterminedbyunpairedStudent'st-testwitha95%confidencelevel.
CalculationofPercentageofIntactRBCs.
Percentpost-thawRBCintegritywascalculatedusingthemeasuredpercenthemolysisvaluesaccordingtothefollowingequation:%post-thawRBCintegrity=100%hemolysis.
Dataisrepresentedasthemeanpercentageofpost-thawRBCintegrityforeachcondition.
Errorbarsarereportedasthestandarderrorofthemean(SEM).
StatisticalsignificanceforalldatawasdeterminedbyunpairedStudent'st-testwitha95%confidencelevel.
Cryomicroscopy.
ThenucleationandgrowthofextracellulariceinsolutionscontainingtheIRIcompoundsweredocumentedusingacryomicroscopethatconsistsofaNikon80ifluorescentmicroscopewithalongwork-ingdistancecondenserandobjectives,CCDcameras(HammamatsuORCA)interfacedtoapersonalcomputerandaconvectioncryomicroscopestage(LinkamFDCS196).
References1.
Sasnoor,L.
M.
,Kale,V.
P.
&Limaye,L.
S.
Supplementationofconventionalfreezingmediumwithacombinationofcatalaseandtrehaloseresultsinbetterprotectionofsurfacemoleculesandfunctionalityofhematopoieticcells.
J.
Hematother.
StemCellRes.
12,553–564(2003).
2.
Pollock,K.
,Sumstad,D.
,Kadidlo,D.
,McKenna,D.
H.
&Hubel,A.
Clinicalmesenchymalstromalcellproductsundergofunctionalchangesinresponsetofreezing.
Cytotherapy17,38–45(2015).
3.
Yoo,K.
H.
,Lee,S.
H.
&Kim,H.
J.
Theimpactofpost-thawcolony-formingunits-granulocyte/macrophageonengraftmentfollowingunrelatedcordbloodtransplantationinpediatricpatients.
BoneMarrowTransplant.
39,515–521(2007).
4.
Wu,L.
etal.
Increasedapoptosisincryopreservedautologoushematopoieticprogenitorcellscollectedbyapheresisanddelayedneutrophilrecoveryaftertransplantation:anestedcase-controlstudy.
Cytotherapy14,205–214(2012).
5.
Sparrow,R.
L.
,Komodromou,H.
,Tippett,E.
,Georgakopoulos,T.
&Xu,W.
ApoptoticlymphocytesandCD34+cellsincryopreservedcordblooddetectedbythefluorescentvitaldyeSYTO16andcorrelationwithlossofL-selectin(CD62L)expression.
BoneMarrowTransplant.
38,61–67(2006).
6.
Page,K.
M.
etal.
Totalcolony-formingunitsareastrong,independentpredictorofneutrophilandplateletengraftmentafterunrelatedumbilicalcordbloodtransplantation:asingle-centeranalysisof435cordbloodtransplants.
Biol.
BloodMarrowTransplant.
17,1362–1374(2011).
7.
Baust,J.
M.
Molecularmechanismsofcellulardemiseassociatedwithcryopreservationfailure.
CellPreserv.
Technol.
1,17–31(2002).
8.
Ben,R.
N.
,Eniade,A.
&Hauer,L.
SynthesisofaC-linkedantifreezeglycoprotein(AFGP)mimic:probesforinvestigatingthemechanismofaction.
Org.
Lett.
1,1759–1762(1999).
9.
Liu,S.
&Ben,R.
N.
C-linkedgalactosylserineAFGPanaloguesaspotentrecrystallizationinhibitors.
Org.
Lett.
7,2385–2388(2005).
10.
Leclere,M.
,Kwok,B.
K.
,Wu,L.
K.
,Allan,D.
S.
&Ben,R.
N.
C-linkedantifreezeglycoprotein(C-AFGP)analoguesasnovelcryoprotectants.
Bioconj.
Chem.
22,1804–1810(2011).
11.
Czechura,P.
,Tam,R.
Y.
,Dimitrijevic,E.
,Murphy,A.
V.
&Ben,R.
N.
TheimportanceofhydrationforinhibitingicerecrystallizationwithC-linkedantifreezeglycoproteins.
J.
Am.
Chem.
Soc.
130,2928–2929(2008).
12.
Tam,R.
Y.
,Ferreira,S.
S.
,Czechura,P.
,Chaytor,J.
L.
&Ben,R.
N.
Hydrationindex–abetterparameterforexplainingsmallmoleculehydrationininhibitionoficerecrystallization.
J.
Am.
Chem.
Soc.
130,17494–17501(2008).
13.
Tam,R.
Y.
etal.
SolutionconformationofC-linkedantifreezeglycoproteinanaloguesandmodulationoficerecrystallization.
J.
Am.
Chem.
Soc.
131,15745–15753(2009).
14.
Capicciotti,C.
J.
etal.
Potentinhibitionoficerecrystallizationbylowmolecularweightcarbohydrate-basedsurfactantsandhydrogelators.
Chem.
Sci.
3,1408–1416(2012).
15.
Balcerzak,A.
K.
,Ferreira,S.
S.
,Trant,J.
F.
&Ben,R.
N.
Structurallydiversedisaccharideanalogsofantifreezeglycoproteinsandtheirabilitytoinhibiticerecrystallization.
Bioorg.
Med.
Chem.
Lett.
22,1719–1721(2012).
16.
Capicciotti,C.
J.
etal.
Smallmoleculeicerecrystallizationinhibitorsenablefreezingofhumanredbloodcellswithreducedglycerolconcentrations.
Nat.
Sci.
Rep.
5,1–10(2015).
17.
Carrell,D.
T.
,Wilcox,A.
L.
&Urry,R.
L.
Effectoffluctuationsintemperatureencounteredduringhandlingandshipmentofhumancryopreservedsemen.
Andrologia28,315–319(1996).
18.
Coelho,P.
,Dobrila,L.
&Rubinstein,P.
(2000).
Effectoftransientwarmingeventsoncellviabilityofplacentalcordblood.
Paperpresentedatthe4thInternationalSymposiumonHematopoieticStemCellTransplantation,UniversityofTokyoMedicalCentre,Tokyo,Japan.
Cytotherapy,3,55–60(2001).
19.
Dobrila,L.
,Coelho,P.
&Rubinstein,P.
Transientwarmingeventsandcellviabilityofplacental/umbilicalcordblood("PCB").
PosterpresentedattheInternationalSocietyofHematotherapyandGraftEngineering(ISHAGE)2001annualmeeting,Quebec,Canada.
CescaTherapeutics:BioarchiveCryostorage(2001).
20.
Harris,D.
T.
etal.
Studiesonpracticalissuesforcordbloodbanking:effectsofionizingradiationandcryopreservationvariables.
OpenStemCellJ.
2,37–44(2010).
21.
Germann,A.
etal.
TemperaturefluctuationsduringdeeptemperaturescryopreservationreducePBMCrecovery,viabilityandT-cellfunction.
Cryobiology67,193–200(2013).
22.
Smith,J.
G.
etal.
Establishingacceptancecriteriaforcell-mediated-immunityassaysusingfrozenperipheralbloodmononuclearcellsstoredunderoptimalandsuboptimalconditions.
Clin.
VaccineImmunol.
14,527–537(2007).
23.
Quintana,A.
B.
etal.
Morphologicalandbiochemicalanalysisofhumancardiacvalveallograftsafterandincrementofthecryostoragetemperature.
Cryobiology59,96–101(2009).
24.
Balcerzak,A.
K.
,Febbraro,M.
&Ben,R.
N.
Theimportanceofhydrophobicmoietiesinicerecrystallizationinhibitors.
RSCAdv.
3,3232–3236(2013).
25.
Mullen,S.
F.
&Critser,J.
K.
Thescienceofcryobiology.
CancerTreat.
Res.
138,83–109(2007).
26.
Mazur,P.
,Leibo,S.
P.
&Chu,E.
H.
Y.
Atwo-factorhypothesisoffreezinginjury:evidencefromchinesehamstertissue-culturecells.
Exp.
CellRes.
71,345–355(1972).
27.
Jackman,J.
etal.
AssessingantifreezeactivityofAFGP8usingdomainrecognitionsoftware.
Biochem.
Biophys.
Res.
Commun.
354,340–344(2007).
28.
Wu,L.
K.
etal.
Carbohydrate-mediatedinhibitionoficerecrystallizationincryopreservedhumanumbilicalcordblood.
Carb.
Res.
346,86–93(2011).
29.
Lane,L.
B.
Freezingpointsofglycerolanditsaqueoussolutions.
Ind.
Eng.
Chem.
Res.
17,924(1925).
30.
Knight,C.
A.
,Hallett,J.
&Devries,A.
L.
Soluteeffectsonicerecrystallization–anassessmenttechnique.
Cryobiology25,55–60(1988).
1031.
Acker,J.
P.
etal.
Aqualitymonitoringprogramforredbloodcellcomponents:invitroqualityindicatorsbeforeandafterimplementationofsemiautomatedprocessing.
Transfusion54,2534–2543(2014).
32.
Drabkin,D.
L.
Thestandardizationofhemoglobinmeasurement.
Am.
J.
Med.
Sci.
217,710–711(1949).
AcknowledgementsTheauthorsgratefullyacknowledgetheNaturalSciencesandEngineeringResearchCouncilofCanada(NSERC),CanadianBloodServices(CBS)andCanadianInstitutesofHealthResearch(CIHR)forfinancialsupport.
Theviewsexpressedhereindonotnecessarilyrepresenttheviewofthefederalgovernment.
J.
G.
B.
thanksCBSforaGraduateFellowshipProgram(GFP)award.
AuthorContributionsR.
N.
B.
andJ.
P.
A.
conceivedoftheexperimentsandJ.
G.
B.
,J.
S.
P.
,T.
R.
T.
andC.
J.
C.
conductedthem.
R.
N.
B.
,J.
G.
BandJ.
S.
P.
wrotethedraftmanuscriptandallauthorscontributedtoediting.
AdditionalInformationCompetingfinancialinterests:Theauthorsdeclarenocompetingfinancialinterests.
Howtocitethisarticle:Briard,J.
G.
etal.
Smallmoleculeicerecrystallizationinhibitorsmitigateredbloodcelllysisduringfreezing,transientwarmingandthawing.
Sci.
Rep.
6,23619;doi:10.
1038/srep23619(2016).
ThisworkislicensedunderaCreativeCommonsAttribution4.
0InternationalLicense.
Theimagesorotherthirdpartymaterialinthisarticleareincludedinthearticle'sCreativeCommonslicense,unlessindicatedotherwiseinthecreditline;ifthematerialisnotincludedundertheCreativeCommonslicense,userswillneedtoobtainpermissionfromthelicenseholdertoreproducethematerial.
Toviewacopyofthislicense,visithttp://creativecommons.
org/licenses/by/4.
0/
Briard1,JessicaS.
Poisson1,TraceyR.
Turner2,ChantelleJ.
Capicciotti1,JasonP.
Acker2&RobertN.
Ben1Duringcryopreservation,icerecrystallizationisamajorcauseofcellulardamage.
Conventionalcryoprotectantssuchasdimethylsulfoxide(DMSO)andglycerolfunctionbyanumberofdifferentmechanismsbutdonotmitigateorcontrolicerecrystallizationatconcentrationsutilizedincryopreservationprocedures.
InNorthAmerica,cryopreservationofhumanredbloodcells(RBCs)utilizeshighconcentrationsofglycerol.
RBCunitsfrozenundertheseconditionsmustbesubjectedtoatime-consumingdeglycerolizationprocessafterthawinginordertoremovetheglycerolto0.
05)as4atonly5mMbutislessIRIactivethanboth3and4sug-gestingthatfactorsotherthanIRIactivitymaybeimportantforthecryoprotectiveactivity.
TheabilitytoreducetheamountofglycerolinthepresenceofanIRIwithoutcompromisingthenumberofRBCsrecoveredissignif-icantbecausecryopreservationusinglessglycerolwillreducepost-thawprocessingtimeintheclinicalsetting.
GiventhattheoptimalconcentrationsforthefreezingofRBCswerenotthesameasthoseutilizedfortheassessmentofIRIactivity(22mM),theIRIactivityof3–5wasre-assessedattheireffectiveinvitroconcentrationsreportedinFig.
4.
ThesedataarepresentedinFig.
5.
Asexpected,theIRIactivityof4doesnotchangedramati-cally,howevericecrystalsizeinthepresenceof110mM3isdramaticallysmallerinsize.
Interestingly,5appearedtobelesssensitivetoconcentrationeffectsasthereislittledifferenceinicecrystalsizedespitethefacttheconcen-trationisapproximatelyfour-foldless.
MeanIceCrystalSizeuponThawingFrozenRBCs.
AnalysisoficecrystalsizeisakeyaspectofthesplatIRIassaythatourlaboratoryhasdeveloped27.
PreviousworkfromourlaboratoryhasdemonstratedthataddingsmallmoleculeIRIsincreasespost-thawviabilityandallowsforareductionintheamountofcryopro-tectants4,10,16,28.
Wepredictedthaticecrystalsshouldbenoticeablysmallerinsizeinthepresenceofanicerecrys-tallizationinhibitor.
Thus,anexperimentwasperformedinwhichhumanRBCswerefrozenusingaLinkamCryostageandtheicewasimagedinthepresenceofcells.
Usingthisapproach,asolutionofRBCsin15%glycerolor15%glycerolwith4(30mM)wascooledatarateof25°C/mintoatemperatureof40°C.
Thesamplewasthenwarmedto10°Catarateof10°C/minandheldfor10minutespriortotakingapicture.
Figure6showstheFigure2.
Chemicalstructureofaryl-glycosides(3,4),aryl-aldonamide(5)andalysine-basednon-ionicsurfactant(6).
Figure3.
IRIactivityofsmallmolecules3–5at22mM.
IRIactivityisrepresentedasapercentmeangrainsize(%MGS)after30minutesofrecrystallizationat6.
4°Ccomparedtoaphosphatebufferedsaline(PBS)positivecontrolforicerecrystallization.
4imagesoficecrystalsina15%glycerolsolutionwithandwithoutRBCs.
Itisimportanttonotethatthepercentageoficeinthe15%glyceroland15%glycerolwith30mM4samplesstaysconstanteveninthepresenceofRBCs.
Inotherwords,thepresenceofRBCsdoesnotappeartoinfluencetheamountoficeinthesample.
However,lessiceisobservedinsamples(withandwithoutRBCs)when4(30mM)ispresentcomparedtothe15%glycerolcon-trol.
Ineachoftheseimages,thepercentageoffrozenfractionissmall.
Thisisbecausetheholdingtemperatureof10°Cisclosetothecolligativefreezingpointdepressionofthe15%glycerolsolution(4°C)andthereforealargefractionofthesampleisunfrozen29.
AstheIRIactivityof3–5wasassessedinconditionswithhighamountsoficepresent,theexperimentwasrepeatedatalowerholdingtemperaturetoensurehighericevolume.
Figure7showsimagesoficecrystalsizewhenRBCswerecooledto40°Catarateof25°C/minandthenwarmedatthesamerateto20°CandheldforFigure4.
OptimizationofIRI(3–5)concentrationforfreezingofhumanRBCsusing15%glycerol.
RBCswereincubatedfor10minuteswith15%glycerolor15%glycerolwithcompound3,4,or5atvariousconcentrations.
Sampleswereheldat5°Cforfiveminutesbeforecontrollednucleationusingforcepspre-cooledinliquidnitrogen.
Thiscontrollednucleationisperformedtoensurethaticenucleationoccursatthesamesub-zerotemperatureof5°Cineachvial.
Thesampleswereheldat5°Cforanadditionalfiveminutesbeforebeingcooledto40°C(1°C/min).
Uponstabilizationat40°C,thesampleswererapidlythawedina37°CwaterbathandthepercentageofintactRBCswasmeasured.
Thesefreezingconditionswererepeatedtwotosixteentimes(n=2–16)foreachfreezingsolution.
Errorbarsarereportedasthestandarderrorofthemean(SEM).
Asterisks(*)indicatesignificantdifferencedeterminedbyunpairedStudent'st-test(pNaNO6,328.
70;found,327.
95.
IceRecrystallizationInhibition(IRI)Activity.
SampleanalysisforIRIactivitywasperformedusingthe"splatcooling"methodaspreviouslydescribed30.
Allcarbohydratederivativesassessedweredissolvedinaphos-phatebufferedsaline(PBS)solutioncomprisedofsodiumchloride(8%w/v),disodiumphosphate(1.
44%w/v),potassiumchloride(0.
2%w/v)andmonopotassiumphosphate(0.
24%w/v)indistilledwateradjustedtopH7.
4withconcentrationhydrochloricacid.
A10μLdropletofthissolutionwasdroppedfromamicropipettethroughatwometerhighplastictube(10cmindiameter)ontoablockofpolishedaluminumpre-cooledtoapproximately80°C.
Thedropletfrozeinstantlyonthepolishedaluminumblockandwasapproximately1cmindiameterand20μmthick.
Thiswaferwasthencarefullyremovedfromthesurfaceoftheblockandtransferredtoacryostageheldat6.
4°Cforannealing.
ItisimportanttonotethatIRIassayshavetypicallyusedannealingtemperaturesrangingfrom4°Cto8°C.
Theannealingtemperatureof6.
4°Cwasutilizedbecausethisisthestandardinourlaboratory.
Afteraperiodof30minutesat6.
4°C,thewaferwasphotographedbetweencrossedpolarizingfiltersusingadigitalcamera(NikonCoolPix5000)fittedtothemicroscope.
Atotalofthreedropsforeachsamplewereassayedandthreeimagesweretakenfromeachwaferwiththeareaoftwelvecrystalsineachimagebeingquantified.
Imageanalysisoftheicewaferswasperformedusingadomainrecognitionsoftware(DRS)program10.
ThisprocessingemployedtheMicrosoftWindowsGraphicalUserInterfacetoallowausertovisuallydemarcateandstoretheverticesoficedomainsinadigitalmicrograph.
Thedatawasthenusedtocal-culatethedomainareas.
AlldatawasplottedandanalyzedusingMicrosoftExcel.
Themeangrain(oricecrystal)size(MGS)ofthesamplewascomparedtotheMGSofthecontrolPBSsolutionforthatsamedayoftesting.
IRIactivityisreportedasthepercentageoftheMGS(%MGS)relativetothePBScontrol.
Therefore,smallpercent-agesrepresentasmallMGS(smallicecrystals),whichisindicativeofhighIRIactivity.
Errorbarsarereportedasthestandarderrorofthemean(SEM).
BloodCollectionandPreparation.
AllRBCunitswereobtainedfromNetCAD(CanadianBloodServices'NetworkCentreforAppliedDevelopment).
Wholebloodwascollectedfromhealthyvolunteersusingstandard-izedphlebotomyguidelinesapprovedbyCanadianBloodServices(CBS).
Informedconsentwasobtainedfromalldonors.
AllexperimentalprotocolswereapprovedbyNetCADandCBS.
EthicsapprovalswereobtainedfromResearchEthicsBoard(REB)atCBSandtheUniversityofAlberta.
Forcryovialexperiments,wholebloodunitswerecollectedandprocessedbyNetCAD(Vancouver,BC).
Thewholebloodwasprocessedusingthebuffycoat(BC)methodtoproduceleukocytereducedSAGMRBCunits,whichhasbeenpreviouslydescribed31.
Forcry-omicroscopyexperiments,wholebloodwascollectedbystandardphlebotomytechniquesintoEDTAcollectiontubes,pooledintoa15mLconicaltubeandthenprocessedtoobtaintheRBCs.
Processingwasachievedbycen-trifugation(10min,4°C,2,200g)followedbyremovaloftheplasmaandBCfractions.
TheremainingRBCswerethenwashedtwicewith0.
9%saline/0.
2%dextrose(SD)followedbyresuspensionoftheRBCsinSDtoafinalhematocritof0.
50L/L.
ThepreparedRBCswereusedonthesamedayofpreparation.
RBCFreezingExperiments.
Thefreezingsolutionconsistsofa30%glycerolsolutionpreparedfromacommerciallyavailableglycerolsolution(57Glycerolyte,Baxter)bydilutingitwith0.
2%/0.
9%dextrose/saline(SD).
Anequalvolumeoffreezingsolutionwasaddedto150μLofRBCsforafinalvolumeof300μL.
Thefinalconcentrationsofallfreezingsolutionswereasindicatedintheresultsanddiscussion.
RBCsuspensionsweretransferredtocryotubesandincubatedatroomtemperaturefor10minutespriortoimmersioninamethanolbathcooledto5°C.
AthermocouplewasinsertedintoaRBC/15%glycerolsample(temperatureprobe)tomonitortemperatureat1secondintervals.
Oncetheinternalsolutionfromthetemperatureprobereached5°C,icenucleationwasinducedbytouchingtheoutsideoftheglasscryotubeswithpre-cooled(inliquidnitrogen)forceps.
Controllednucleationisperformedtoensurethaticenucleationoccursatthesamesub-zerotemperatureof5°Cineachvial.
RBCsampleswerethenheldat5°Cfor5minutes.
Sampleswerethencooledatarateof1°C/minto40°C,thenthawedimmediatelybyplungingina37°Cwaterbath.
Post-thawhematocrits(Hcts)andpercenthemolysiswasdeterminedforallfreezingexperimentsbycomparingthesupernatanthemoglobinconcentrationtototalhemoglobinconcentrationusingthecyanmethemoglobinDrabkin'smethod32.
Thesefreez-ingconditionswererepeatedtwotosixteentimes(n=2–16)foreachfreezingsolution.
PercentageofintactRBCswasgraphedinadditiontoerrorbarsreportedasthestandarderrorofthemean(SEM).
StatisticalsignificanceforalldatawasdeterminedbyunpairedStudent'st-testwitha95%confidencelevel.
TransientWarmingExperiments.
Thefreezingsolutionconsistsofa30%glycerolsolutionpreparedfromacommerciallyavailableglycerolsolution(57Glycerolyte,Baxter)bydilutingitwith0.
2%/0.
9%dextrose/saline(SD).
Anequalvolumeoffreezingsolutionwasaddedto150μLofRBCsforafinalvolumeof300μL.
Thefinalconcentrationsofallfreezingsolutionswereasindicatedintheresultsanddiscussion.
RBCsuspensionsweretransferredtocryotubesandincubatedatroomtemperaturefor10minutespriortoimmersionindryice(80°C).
Atemperatureprobewasusedfortemperaturemeasurementsat1secondintervals.
Oncetheinternalsolutionreached80±2°C,thesampleswereimmersedinamethanolbathcooledto20°C.
Oncetheinternalsolutionreached20°C,thesampleswereplungedintodryiceagain.
RBCsampleswereheldindryiceuntiltheinternalsolutionfromthetemperatureprobereached80±2°C,afterwhichthesampleswereeitherthawed(representingonecycleoftransientwarming)orimmersedinamethanolbathcooledto20°C.
One,threeandfivecyclesofimmersionina20°Cmethanolbathanddryicewereperformed.
Sampleswerethawedquicklybyplungingina37°Cwaterbath.
Post-thawHctsandpercenthemolysiswasdeterminedforallfreezingexper-imentsbycomparingthesupernatanthemoglobinconcentrationtototalhemoglobinconcentrationusingthe9cyanmethemoglobinDrabkin'smethod32.
Thesefreezingconditionswererepeatedtwotosixtimes(n=2–6)foreachfreezingsolution.
PercentageofintactRBCswasgraphedinadditiontoerrorbarsreportedasthestandarderrorofthemean(SEM).
StatisticalsignificanceforalldatawasdeterminedbyunpairedStudent'st-testwitha95%confidencelevel.
CalculationofPercentageofIntactRBCs.
Percentpost-thawRBCintegritywascalculatedusingthemeasuredpercenthemolysisvaluesaccordingtothefollowingequation:%post-thawRBCintegrity=100%hemolysis.
Dataisrepresentedasthemeanpercentageofpost-thawRBCintegrityforeachcondition.
Errorbarsarereportedasthestandarderrorofthemean(SEM).
StatisticalsignificanceforalldatawasdeterminedbyunpairedStudent'st-testwitha95%confidencelevel.
Cryomicroscopy.
ThenucleationandgrowthofextracellulariceinsolutionscontainingtheIRIcompoundsweredocumentedusingacryomicroscopethatconsistsofaNikon80ifluorescentmicroscopewithalongwork-ingdistancecondenserandobjectives,CCDcameras(HammamatsuORCA)interfacedtoapersonalcomputerandaconvectioncryomicroscopestage(LinkamFDCS196).
References1.
Sasnoor,L.
M.
,Kale,V.
P.
&Limaye,L.
S.
Supplementationofconventionalfreezingmediumwithacombinationofcatalaseandtrehaloseresultsinbetterprotectionofsurfacemoleculesandfunctionalityofhematopoieticcells.
J.
Hematother.
StemCellRes.
12,553–564(2003).
2.
Pollock,K.
,Sumstad,D.
,Kadidlo,D.
,McKenna,D.
H.
&Hubel,A.
Clinicalmesenchymalstromalcellproductsundergofunctionalchangesinresponsetofreezing.
Cytotherapy17,38–45(2015).
3.
Yoo,K.
H.
,Lee,S.
H.
&Kim,H.
J.
Theimpactofpost-thawcolony-formingunits-granulocyte/macrophageonengraftmentfollowingunrelatedcordbloodtransplantationinpediatricpatients.
BoneMarrowTransplant.
39,515–521(2007).
4.
Wu,L.
etal.
Increasedapoptosisincryopreservedautologoushematopoieticprogenitorcellscollectedbyapheresisanddelayedneutrophilrecoveryaftertransplantation:anestedcase-controlstudy.
Cytotherapy14,205–214(2012).
5.
Sparrow,R.
L.
,Komodromou,H.
,Tippett,E.
,Georgakopoulos,T.
&Xu,W.
ApoptoticlymphocytesandCD34+cellsincryopreservedcordblooddetectedbythefluorescentvitaldyeSYTO16andcorrelationwithlossofL-selectin(CD62L)expression.
BoneMarrowTransplant.
38,61–67(2006).
6.
Page,K.
M.
etal.
Totalcolony-formingunitsareastrong,independentpredictorofneutrophilandplateletengraftmentafterunrelatedumbilicalcordbloodtransplantation:asingle-centeranalysisof435cordbloodtransplants.
Biol.
BloodMarrowTransplant.
17,1362–1374(2011).
7.
Baust,J.
M.
Molecularmechanismsofcellulardemiseassociatedwithcryopreservationfailure.
CellPreserv.
Technol.
1,17–31(2002).
8.
Ben,R.
N.
,Eniade,A.
&Hauer,L.
SynthesisofaC-linkedantifreezeglycoprotein(AFGP)mimic:probesforinvestigatingthemechanismofaction.
Org.
Lett.
1,1759–1762(1999).
9.
Liu,S.
&Ben,R.
N.
C-linkedgalactosylserineAFGPanaloguesaspotentrecrystallizationinhibitors.
Org.
Lett.
7,2385–2388(2005).
10.
Leclere,M.
,Kwok,B.
K.
,Wu,L.
K.
,Allan,D.
S.
&Ben,R.
N.
C-linkedantifreezeglycoprotein(C-AFGP)analoguesasnovelcryoprotectants.
Bioconj.
Chem.
22,1804–1810(2011).
11.
Czechura,P.
,Tam,R.
Y.
,Dimitrijevic,E.
,Murphy,A.
V.
&Ben,R.
N.
TheimportanceofhydrationforinhibitingicerecrystallizationwithC-linkedantifreezeglycoproteins.
J.
Am.
Chem.
Soc.
130,2928–2929(2008).
12.
Tam,R.
Y.
,Ferreira,S.
S.
,Czechura,P.
,Chaytor,J.
L.
&Ben,R.
N.
Hydrationindex–abetterparameterforexplainingsmallmoleculehydrationininhibitionoficerecrystallization.
J.
Am.
Chem.
Soc.
130,17494–17501(2008).
13.
Tam,R.
Y.
etal.
SolutionconformationofC-linkedantifreezeglycoproteinanaloguesandmodulationoficerecrystallization.
J.
Am.
Chem.
Soc.
131,15745–15753(2009).
14.
Capicciotti,C.
J.
etal.
Potentinhibitionoficerecrystallizationbylowmolecularweightcarbohydrate-basedsurfactantsandhydrogelators.
Chem.
Sci.
3,1408–1416(2012).
15.
Balcerzak,A.
K.
,Ferreira,S.
S.
,Trant,J.
F.
&Ben,R.
N.
Structurallydiversedisaccharideanalogsofantifreezeglycoproteinsandtheirabilitytoinhibiticerecrystallization.
Bioorg.
Med.
Chem.
Lett.
22,1719–1721(2012).
16.
Capicciotti,C.
J.
etal.
Smallmoleculeicerecrystallizationinhibitorsenablefreezingofhumanredbloodcellswithreducedglycerolconcentrations.
Nat.
Sci.
Rep.
5,1–10(2015).
17.
Carrell,D.
T.
,Wilcox,A.
L.
&Urry,R.
L.
Effectoffluctuationsintemperatureencounteredduringhandlingandshipmentofhumancryopreservedsemen.
Andrologia28,315–319(1996).
18.
Coelho,P.
,Dobrila,L.
&Rubinstein,P.
(2000).
Effectoftransientwarmingeventsoncellviabilityofplacentalcordblood.
Paperpresentedatthe4thInternationalSymposiumonHematopoieticStemCellTransplantation,UniversityofTokyoMedicalCentre,Tokyo,Japan.
Cytotherapy,3,55–60(2001).
19.
Dobrila,L.
,Coelho,P.
&Rubinstein,P.
Transientwarmingeventsandcellviabilityofplacental/umbilicalcordblood("PCB").
PosterpresentedattheInternationalSocietyofHematotherapyandGraftEngineering(ISHAGE)2001annualmeeting,Quebec,Canada.
CescaTherapeutics:BioarchiveCryostorage(2001).
20.
Harris,D.
T.
etal.
Studiesonpracticalissuesforcordbloodbanking:effectsofionizingradiationandcryopreservationvariables.
OpenStemCellJ.
2,37–44(2010).
21.
Germann,A.
etal.
TemperaturefluctuationsduringdeeptemperaturescryopreservationreducePBMCrecovery,viabilityandT-cellfunction.
Cryobiology67,193–200(2013).
22.
Smith,J.
G.
etal.
Establishingacceptancecriteriaforcell-mediated-immunityassaysusingfrozenperipheralbloodmononuclearcellsstoredunderoptimalandsuboptimalconditions.
Clin.
VaccineImmunol.
14,527–537(2007).
23.
Quintana,A.
B.
etal.
Morphologicalandbiochemicalanalysisofhumancardiacvalveallograftsafterandincrementofthecryostoragetemperature.
Cryobiology59,96–101(2009).
24.
Balcerzak,A.
K.
,Febbraro,M.
&Ben,R.
N.
Theimportanceofhydrophobicmoietiesinicerecrystallizationinhibitors.
RSCAdv.
3,3232–3236(2013).
25.
Mullen,S.
F.
&Critser,J.
K.
Thescienceofcryobiology.
CancerTreat.
Res.
138,83–109(2007).
26.
Mazur,P.
,Leibo,S.
P.
&Chu,E.
H.
Y.
Atwo-factorhypothesisoffreezinginjury:evidencefromchinesehamstertissue-culturecells.
Exp.
CellRes.
71,345–355(1972).
27.
Jackman,J.
etal.
AssessingantifreezeactivityofAFGP8usingdomainrecognitionsoftware.
Biochem.
Biophys.
Res.
Commun.
354,340–344(2007).
28.
Wu,L.
K.
etal.
Carbohydrate-mediatedinhibitionoficerecrystallizationincryopreservedhumanumbilicalcordblood.
Carb.
Res.
346,86–93(2011).
29.
Lane,L.
B.
Freezingpointsofglycerolanditsaqueoussolutions.
Ind.
Eng.
Chem.
Res.
17,924(1925).
30.
Knight,C.
A.
,Hallett,J.
&Devries,A.
L.
Soluteeffectsonicerecrystallization–anassessmenttechnique.
Cryobiology25,55–60(1988).
1031.
Acker,J.
P.
etal.
Aqualitymonitoringprogramforredbloodcellcomponents:invitroqualityindicatorsbeforeandafterimplementationofsemiautomatedprocessing.
Transfusion54,2534–2543(2014).
32.
Drabkin,D.
L.
Thestandardizationofhemoglobinmeasurement.
Am.
J.
Med.
Sci.
217,710–711(1949).
AcknowledgementsTheauthorsgratefullyacknowledgetheNaturalSciencesandEngineeringResearchCouncilofCanada(NSERC),CanadianBloodServices(CBS)andCanadianInstitutesofHealthResearch(CIHR)forfinancialsupport.
Theviewsexpressedhereindonotnecessarilyrepresenttheviewofthefederalgovernment.
J.
G.
B.
thanksCBSforaGraduateFellowshipProgram(GFP)award.
AuthorContributionsR.
N.
B.
andJ.
P.
A.
conceivedoftheexperimentsandJ.
G.
B.
,J.
S.
P.
,T.
R.
T.
andC.
J.
C.
conductedthem.
R.
N.
B.
,J.
G.
BandJ.
S.
P.
wrotethedraftmanuscriptandallauthorscontributedtoediting.
AdditionalInformationCompetingfinancialinterests:Theauthorsdeclarenocompetingfinancialinterests.
Howtocitethisarticle:Briard,J.
G.
etal.
Smallmoleculeicerecrystallizationinhibitorsmitigateredbloodcelllysisduringfreezing,transientwarmingandthawing.
Sci.
Rep.
6,23619;doi:10.
1038/srep23619(2016).
ThisworkislicensedunderaCreativeCommonsAttribution4.
0InternationalLicense.
Theimagesorotherthirdpartymaterialinthisarticleareincludedinthearticle'sCreativeCommonslicense,unlessindicatedotherwiseinthecreditline;ifthematerialisnotincludedundertheCreativeCommonslicense,userswillneedtoobtainpermissionfromthelicenseholdertoreproducethematerial.
Toviewacopyofthislicense,visithttp://creativecommons.
org/licenses/by/4.
0/
- cryostagenano6相关文档
- m3nano6
- rangenano6
- Lys162nano6
- 2013年5月1日更新(111篇Highly
- actionnano6
- plansnano6
Boomer.Host(年付3.5美)休斯敦便宜VPS
Boomer.Host是一家比较新的国外主机商,虽然LEB自述 we’re now more than 2 year old,商家提供虚拟主机和VPS,其中VPS主机基于OpenVZ架构,数据中心为美国得克萨斯州休斯敦。目前,商家在LET发了两款特别促销套餐,年付最低3.5美元起,特别提醒:低价低配,且必须年付,请务必自行斟酌确定需求再入手。下面列出几款促销套餐的配置信息。CPU:1core内存:...
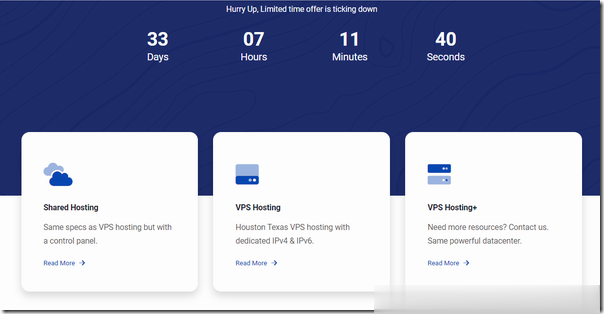
A2Hosting三年付$1.99/月,庆祝18周年/WordPress共享主机最高优惠81%/100GB SSD空间/无限流量
A2Hosting主机,A2Hosting怎么样?A2Hosting是UK2集团下属公司,成立于2003年的老牌国外主机商,产品包括虚拟主机、VPS和独立服务器等,数据中心提供包括美国、新加坡softlayer和荷兰三个地区机房。A2Hosting在国外是一家非常大非常有名气的终合型主机商,拥有几百万的客户,非常值得信赖,国外主机论坛对它家的虚拟主机评价非常不错,当前,A2Hosting主机庆祝1...
印象云七夕促销,所有机器7折销售,美国CERA低至18元/月 年付217元!
印象云,成立于2019年3月的商家,公司注册于中国香港,国人运行。目前主要从事美国CERA机房高防VPS以及香港三网CN2直连VPS和美国洛杉矶GIA三网线路服务器销售。印象云香港三网CN2机房,主要是CN2直连大陆,超低延迟!对于美国CERA机房应该不陌生,主要是做高防服务器产品的,并且此机房对中国大陆支持比较友好,印象云美国高防VPS服务器去程是163直连、三网回程CN2优化,单IP默认给20...
nano6为你推荐
-
vps汽车的VPS是什么,和GPS有什么区别域名购买在网上购买域名 会受骗吗万网虚拟主机如何购买万网的虚拟主机?云南虚拟主机云南服务器托管广西虚拟主机怎样建立虚拟机和本地计算机的桥接四川虚拟主机哪些网站适合租用独立服务器?双线虚拟主机什么是智能双线虚拟主机?联动天下的双线主机有什么优势?买域名买域名的时候需要那些注意?域名拍卖我发现我自己的域名在拍卖。我应该怎么做?子域名查询爱站网外链查询工具