sitesyw372
yw372:Com 时间:2021-04-11 阅读:()
ProductionofbiodieselfromJatrophaoilcatalyzedbynanosizedsolidbasiccatalystXinDenga,ZhenFanga,*,Yun-huLiua,Chang-LiuYubaBiomassGroup,XishuangbannaTropicalBotanicalGarden,ChineseAcademyofSciences,88Xufulu,Kunming,Yunnanprovince650223,ChinabPerochemicalCompany,DagangOileldGroup,Tianjin300280,ChinaarticleinfoArticlehistory:Received2November2010Receivedinrevisedform17December2010Accepted17December2010Availableonline19January2011Keywords:JatrophabiodieselNanoparticlesSolidcatalystHydrotalciteabstractInthiswork,hydrotalcite-derivedparticleswithMg/Almolarratioof3/1weresynthesizedbyacopre-cipitationmethodusingureaasprecipitatingagent,subsequentlywith(MHT)microwave-hydrothermaltreatment,andfollowedbycalcinationat773Kfor6h.
Theseparticlesweremicro-sizedmixedMg/AloxidesascharacterizedbySEMandAFM.
ButactuallytheywerenanosizedaccordingtothecalculationsfromXRDdata.
Becauseoftheirstrongbasicity,thenanoparticleswerefurtherusedascatalystforbiodieselproductionfromJatrophaoilafterpretreatment.
Experimentswereconductedwiththesolidbasiccatalystinanultrasonicreactorunderdifferentconditions.
Attheoptimizedcondition,biodieselyieldof95.
2%wasachieved,andthebiodieselpropertieswereclosetothoseoftheGermanstandard.
Thecatalystcanbereusedfor8times.
2010ElsevierLtd.
Allrightsreserved.
1.
IntroductionBiodieselhasdrawnmoreandmoreattentioninrecentyearsbecauseitisrenewableandhaslessdetrimentaleffectsonenvi-ronmentascomparedwithconventionaldieselderivedfrompetroleum[1].
Biodieselobtainedfromrenewablebiomassfeed-stockscanbeusedindieselenginesorblendedatvariousproportionswithpetroleumdieselasfuel[2].
Itconsistsofmono-alkylestersthatareusuallyproducedbytransestericationofplantoilwithmethanolorethanol.
Ithassimilarandsometimesbetterphysicalandchemicalpropertiesthanpetroleumdiesel,suchashigherashpoint,ultra-lowersulphurconcentration,betterlubricatingefciencyandfewpollutantsproduced[3e6].
However,biodieselisexpensiveduetothehighpriceofplantoilandsomeprocessingtechnologicalissues,suchascatalystandequipment.
Therefore,littlecommercialbiodieselisusedinChina.
Therawmaterialsexploitedcommerciallybysomedevelopedcountriesareedibleoilssuchas,rapeseed,soybean,palm,sunower,coconutandlinseedoils[3,7].
Thefractionofrawmaterialsforworldcommercialbiodieselproductionisrapeseedoil84%,sunoweroil13%,palmoil1%,soybeanoilandothers2%[7].
UsingedibleoilstoproducebiodieselindevelopingcountriessuchasChinawithlimitedarablelandpercapitaisnotfeasibleandisbanned.
Therefore,onlynon-edibleplantsareconsideredasfavorableresourcesforbiodieselproduction,suchasChinesetallow[8]andJatrophacurcasL.
trees[9,10],whichareproducingnon-edibleoilinappreciablequantityandcanbegrowninalarge-scaleonnon-croppedmarginallandsandwastelands.
JatrophacurcasL.
,adrought-resistantshrubortree,iswidelydistributedinthewildinsemi-cultivatedtropicalorsubtropicalareasinCentralandSouthAmerica,Africa,India,andSouthChina.
Ithasaproductivelifespaninexcessof30years.
ThefattyacidcompositionofJatrophaoilissimilartootheredibleoils,butthepresenceofsometoxicmaterialsinkernel(e.
g.
,curcin)renderstheoilunsuitableforcookingpurposes[11,12].
TheoilcontentinJatrophaseedrangesfrom25%to40%byweightandinthekernelitselfrangesfrom45%to60%[10].
Nowadays,Jatrophatrees,asapotentialalternativebiodieselcrop,arewidelycultivatedinSouthwestofChinasuchasYunnan,Sichuan,andGuangxiprovinces[9].
Inthenearfuture,itwillsupplypartofcrudeoilforcommercialbiodieselproductioninChina.
TheconventionalindustrialproductionofbiodieselisthroughtransestericationofcrudeoilwithahomogeneousstrongbasecatalystsuchasNaOHandKOH[1,3,13].
Afterreaction,recoveryofglycerol,removalofbasecatalystfromproducts,andtreatmentofalkalinewastewaterarecostlyandnon-environmental.
Further-more,ahomogeneousbasecatalystisineffectiveforproductionbiodieselfromhighacid-valuecrudeoilsduetotheformationofsoap.
Tanetal.
[14]reportedacatalyst-freebiodieselproductionmethod,usingwastepalmcookingoilasrawmaterialand*Correspondingauthor.
Tel.
:868715163360;fax:868715160916.
E-mailaddress:zhenfang@xtbg.
ac.
cn(Z.
Fang).
URL:http://brg.
groups.
xtbg.
cn/ContentslistsavailableatScienceDirectEnergyjournalhomepage:www.
elsevier.
com/locate/energy0360-5442/$eseefrontmatter2010ElsevierLtd.
Allrightsreserved.
doi:10.
1016/j.
energy.
2010.
12.
043Energy36(2011)777e784supercriticalmethanol.
Butthemethodhasahighcostinreactorandoperation(duetohighpressuresandhightemperatures),andhighmethanolconsumption(e.
g.
,highmethanol/crude-oilmolarratioof40/1).
Therefore,heterogeneoussolidcatalystswereusedforthetransestericationprocess,whereabetterseparationandreuseofthecatalystswithoutsaponicationwereachieved[15].
Preparationandapplicationofsolidsuperbasecatalystsareanemergingareathatisattractingmoreandmoreattention,becausethecatalystsareeasilyseparatedforreuseandpossessahighactivityforvariousreactionsundermildconditions.
Theycanreplacehomogeneousbasecatalystsinordertominimizetheproductionofpollutants.
LDHs(Layereddoublehydroxides)areimportantinorganicmaterialswithlayeredstructureandanionexchangedcapacity.
Asclassicalsolidbasematerials,calcinedLDHsarewidelyusedascatalystintheproductionofbiodiesel[16e18].
Inpreviouswork[17],transestericationprocesswascarriedoutwithreuxofmethanol,methanol/soybean-oilmolarratioof15/1,reactiontimeof9handcatalystamountof7.
5%,andoilconversionratewasonly67%.
IntheworkofBritoetal.
[18],wasteoilasfeedstock,biodieselproductionwasperformedathightempera-turesrangingfrom353to433K,methanol/oilmolarratiofrom12/1to48/1andcatalystconcentrationfrom3to12%,respectively,and90%biodieselyieldwasachieved.
Ultrasonicradiationisarelativenewtechniquethatresultsintheformationandcollapseofmicro-scalebubblesinliquidtogeneratelocalhightemperatureandhighpressure.
So,itcanbeusedasanalternativeenergysourcetopromotereactions.
Itenormouslyreducesreactiontime,methanol/oilmolarratioandcatalystconcentration.
Therefore,biodieselproductionwithcalcinedhydrotalcitecatalystcoupledwithultra-sonicradiationislikelytobecomeaneweffectivemethod.
Thepurposeofthisworkistosynthesizehydrotalciteparticlesandsubsequentlytocalcinethemascatalystforbiodieselproduc-tion.
HydrotalciteparticleswerepreparedinanultrasonicreactorbycoprecipitationofaqueousnitratesolutionsofMgandAlusingureaasprecipitatingagent,andsubsequentlywithMHT(micro-wave-hydrothermaltreatment).
Thesynthesizedparticles,aftercalcination,wereusedassolidbasiccatalystinthetrans-estericationofJatrophaoiltotesttheiractivitiesintheultrasonicreactor.
Experimentsunderdifferentreactionconditions,suchasmethanol/Jatropha-oilmolarratio,catalystconcentration,reactiontemperatureandultrasonicpower,wereperformedinordertooptimizebiodieselyield.
Thereasonsforcatalystdeactivationwerealsostudied.
2.
Experimental2.
1.
MaterialsNitratesofMgandAl(99.
8%purity)usedinthesynthesiswereobtainedfromChengdouFineChemicalCo.
Ltd.
Urea(99.
0%purity)andmethanol(99.
0%purity)werepurchasedfromShanghaiFineChemicalCo.
Ltd.
JatrophaoilwithhighFFAs(free-fatty-acids)wasobtainedfromourinstituteinXishuangbanna,Yunnanprovince,SouthwestofChina[9].
AccordingtotheChineseNationalstan-dardsGB9014.
2-88andGB164-64,AV(acidvalue)andSV(saponicationvalue)oftheJatrophaoilweremeasuredas10.
5mgKOH/gand191.
0mgKOH/g,respectively.
Soitsmolecularweightwascalculatedas932g/molbytheformula:M(56.
110003)/(SVAV)[19].
2.
2.
CatalystpreparationHydrotalciteparticleswithMg/Almolarratioof3/1weresynthesizedbyacoprecipitationmethodusingureaasprecipitatingagent,andwithMHT.
Inthismethod,asolution(0.
15MMgnitrateand0.
05MAlnitrate)wasmadeinathree-neckaskbydissolvingthetwosolidnitratesindeionizedwater.
Urea{[urea]/[NO3]molarratioof4/1}wasthenaddedintotheask.
Theaskwassubmergedinanoilbathat373Kwithstirringabout500rpmintheultrasonicreactorfor2h.
TheslurryobtainedwassubsequentlysubmittedtoMHTinthemotherliquorfor2hat393K.
Afterltered,washedthoroughlywithdeionizedwateranddriedat373Kfor10hatavacuumcondition,solidhydrotalciteparticleswereobtained.
TheywerecharacterizedbypowderXRD(X-RayDiffraction).
Catalyticactivityofhydrotalcitewasstudiedbythetrans-estericationofJatrophaoilwithmethanoltobiodiesel.
Withoutcalcination,hydrotalcitedisplayednoparticularcatalyticactivityinthereaction.
But,aftercalcined,hydrotalcitewasintheformofOHcontainedmixedAl-Mgoxideswhichhadheterogeneoussurfacebasicitywithbasicsitesoflow(OH-groups),medium(Mg-Opairs),andstrong(O2anions)basicityandexhibitedasignicantactivity[20].
So,hydrotalciteparticleswerecalcinedat773Kfor6hinamufefurnaceandusedascatalystforthefollowingbiodieselproduction.
2.
3.
BiodieselproductionBiodieselproductionprocessisstronglyinuencedbythefattyacidcompositionandFFAscontentofcrudeoil.
TheAVoffreshJatrophaoilinXishuangbanna,Yunnanprovince,isfrom5to12mgKOH/g.
OwingtoitshighFFAs,thetransestericationofJatrophaoiltobiodieselcatalyzeddirectlybysolidcatalysthadalowconver-sionrate.
Evenusinglongerreactiontime,only80.
4%biodieselyieldwasachieved.
Inordertoincreasebiodieselyield,FFAsinJatrophaoilneedtoremove.
Atwo-stepprocessofJatrophaoiltobiodieselwasusedtoresolvetheproblem[10].
First,anacid-estericationpretreatmentwasperformedwithconcentratedsulfuricacidascatalyst.
Thethree-neckaskwithawater-cooledcondenserwaslledwith200-mLJatrophaoil,40-mLanhydrousmethanoland4-mLsulfuricacid.
Themixturewasvigorouslystirredandreuxedat318Kinanultrasonicreactorfor1.
5h.
Afterreaction,themixturewasltered,andtheunreactedmethanolwasseparatedfromtheliquidphaseviarotaryevapo-ration.
Theoilwaswashedthreetimeswithsodiumchloridesolutionthendriedusinganhydroussodiumsulfate.
Afterthepretreatment,theAVofthepretreatedoilwasreducedtoonly0.
7mgKOH/gwith93.
3%estericationrate.
Thereactionequationwasdescribedasbelow:RCOOHCH3OH/RCOOCH3H2O(1)Thesecondstep,abase-catalyzedtransestericationreaction(Eq.
(2))wascarriedoutinthesameultrasonicreactorwiththecalcinedhydrotalcitecatalyst:CH2OOCR1jCHOOCR2jCH2OOCR33CH3OH#CatalystCH2OHjCHOHjCH2OHR1COOCH3jR2COOCH3jR3COOCH3(2)Biodieselyieldwascalculatedas:Biodieselyield%totalactualweightofmethylesters100=totaltheoreticalweightofmethylesters3where,totalweightofmethylesterswereproducedfrombothsteps,totaltheoreticalweightwasobtainedassuming100%conversionoffattyacidsinJatrophaoilaccordingtoEqs.
1and2.
Thereactionprocedurewasasfollows:rst,thecatalystwasdispersedinmethanolwithmechanicalstirring(about600rpm).
X.
Dengetal.
/Energy36(2011)777e784778Then,theabovepretreatedoilwasaddedintothemixtureandheatedto318Kbywaterbath.
Afterreaction,excessivemethanolwasdistilledoffunderavacuumconditionwhilecatalystwasseparatedbyhigh-speedcentrifugation.
Afterremovaloftheglyc-erollayer,biodieselwascollectedandreadyforChromatographicanalyses.
2.
4.
Jatrophaoilandbiodieselanalyses2.
4.
1.
JatrophaoilThecompositionofcrudeoilwasanalyzedbyGasChromatog-raphy(GC,Shimadzu,GC-2014)withaameionizationdetectorandacapillarycolumn(Rtx-Wax30m0.
25mm0.
25mm).
Oxygen-freenitrogenwasusedasacarriergasataowrateof1.
4mL/min.
Otherconditionswereasfollows:initialoventemperatureof443K(2min),rampat5.
0K/min,naltemperatureof503K(4min),injectortemperatureof523K,detectortemper-atureof553K,andasplitratioof39/1.
Theanalysiswascarriedoutbyinjecting1-mLsamplesolution(0.
25-mLcrudeJatropha-oildis-solvedin9.
75-mLn-hexane)intotheGC.
Undecanoicacidmethylesterwasusedastheinternalstandard.
ThefattyacidsinJatrophaoilwereidentiedandquantiedbycomparingtheirretentiontimesandpeakareastothoseofstandardfattyacids.
Fig.
1showssixfattyacidswereidentiedandquantiedas:palmiticacid(C16:0)15.
18%,palmitoleicacid(C16:1)0.
99%,stearicacid(C18:0)6.
25%,oleicacid(C18:1)41.
17%,linoleicacid(C18:2)31.
25%,andlinolenicacid(C18:3)0.
08%.
2.
4.
2.
BiodieselBiodieselwasalsoanalyzedbytheaboveGCmethodundersimilarconditions.
Thepreparedbiodiesel(5mL)wasdissolvedin20-mLdichloromethaneand1-mLinternalstandardsolutionforGCanalysis.
Samplesolutions(1mL)wereinjectedbyasampleratanoventemperatureof493K.
Heliumwasusedascarriergasataowrateof0.
8mL/min.
Identicationofmethylesterpeakswasdonebycomparingtheretentiontimesbetweenthesamplesandthestandardcompounds.
Methylesters(biodiesel)werequantiedbycomparingthepeakareasbetweenthesamplesandthestan-dardcompounds.
2.
5.
CharacterizationofhydrotalciteparticlesXRDanalyseswereconductedonaSiemensD-5000diffrac-tometerwithaCuKaradiationsourceatvoltageof40kVandcurrentof30mA,Ni-lteredandagraphitesecondarymono-chromater.
Sampleswereanalyzedinacontinuousscanmodebetween5and70(2q).
ScanningElectronMicroscope(SEM,HitachiS-520)measurementswerecarriedoutat11kV.
AFM(AtomicForceMicroscope)imageswereobtainedusingWET-SPM-9500-J3instrument(ShimadzuCo.
Ltd.
).
SamplesforAFMobser-vationweredispersedinanalcoholsolutionfor8hinanultrasonicreactor.
NitrogenphysisorptionwasconductedonaMicromeriticsASAP-2020surfaceareaandporosimetrysystem.
TheelementalanalysesweredeterminedbywavelengthdispersiveXRF(X-RayFluorescence)spectrometer(BrukerAXS).
2.
6.
PhysicochemicalpropertiesofbiodieselFlashpoint,pourpoint,viscosity,AVandcetanenumberofbiodieselweremeasuredaccordingtotheChinesenationalstan-dardsGB267-64,GB265-64,GB164-64andGB386-64.
Otherphysicochemicalpropertiesofbiodieselwereobtainedaccordingtoreferences[19]and[21].
3.
Resultsanddiscussion3.
1.
CharacterizationofhydrotalciteandcalcinedhydrotalciteXRDpatternsofhydrotalciteparticleswithMg/Almolarratioof3/1weregiveninFig.
2.
InFig.
2a,theparticlesexhibitedasinglephase,correspondingtoatypicalhydrotalcitestructure(JCPDSle70-2151)withstrong,sharp,andsymmetricpeaksforthe(003),(006),(110),and(113)planesaswellasbroadandsymmetricpeaksforthe(009),(015),and(018)planes[22].
Theaverageparticlesizewascalculatedas7.
3nmbytheSchererequation[Dck((l/b)cosq);whereDcistheaverageparticlesize;kistheSchererconstant(0.
89);listheX-raywavelength,(CuKa)0.
1541nm;bistheFWHM(full-widthathalf-maximum);qisthediffractionangle]intheXRD(003)reection[23,24].
Aftercalcinedat773Kfor6h,hydrotalciteparticlesweredecomposedtomixedMg-Alcomplexoxides,whichwereconrmedbytheXRDpatterninFig.
2b.
Forthecalcinedparticles,thecharacteristicreectionswereobservedclearlyat2qof43and63,correspondingtoanMgO-likephaseormagnesia-aluminasolidphase.
ThepeaksofAl2O3phasewereverysmall,indicatingthatAl3cationsweredispersedinthestructureofMgOwithouttheformationofspinelspecies[15,25].
SEMimagesofhydrotalciteandcalcinedhydrotalciteweregiveninFig.
3.
HydrotalcitehadarelativelyuniformhexagonalFig.
1.
GCgraphforcrudeJatrophaoil.
Fig.
2.
XRDpatternsofhydrotalcite:(a)withoutcalcination,and(b)aftercalcinedat773Kfor6h.
X.
Dengetal.
/Energy36(2011)777e784779platelet-likestructure,butpartlydestroyedatthecalcinedtemperatureof773K.
Beforecalcination,hydrotalcitehadawell-developedplateletstructureofatypicallayeredmaterialwithauniformsizeof1mmwidthand72nmthickness(Fig.
3A)thatismuchlargerthantheabovecalculatedvalue(7.
3nm).
Thepossiblereasonisthattheplateletswereformedbyagglomerationwithnumerousnenanoparticles.
Aftercalcinedat773K,theuniformhexagonalplateletstructurewaspartlydestroyedduetotheremovalofhydroxylgroupsinthemetalhydroxidelayersandcarbondioxidesproductionfromthedecompositionofCO32existedintheinterlayerspaceascharge-balancinganion.
Butitstillkeptsheetstructurewithasmallersizeof372nmwidthand72nmthickness(Fig.
3B).
AFMimagesofthecalcinedhydrotalciteparticlesat773Kfor6hwiththesolidstructure,diameterandthicknessweregiveninFig.
4.
Theparticlesofthemetalhydroxidelayerswerecongregatedtoformalargelayeredstructurewiththesizeof941nmwidthand381nmthickness.
However,theaverageparticlesizewasonly372nmwidthand72nmthicknessinFig.
3B,whichindicatedthatlargeparticleswereformedbyagglomerationthatwerehardtoseparateforAFManalysesevenafterultrasonicdispersiontreatment.
TheMg/AlatomicratiointhemixedmetaloxidesamplewasveriedbyXRFspectroscopy.
TheactualMg/Alatomicratiowas2.
78/1,thatiscloseto3/1ofthestartingMgandAlnitrates.
Phys-ical-chemicalpropertiesofcalcinedhydrotalciteweregiveninTable1,andtheyagreedwellwiththeearliermeasurementsbyTantir-ungrotechaietal.
[16].
TheMg-Almixedmetaloxidesampleexhibitedhighsurfaceareaof218m2/gwithporevolumeandporediameterof0.
17cm3/gand3.
9nm,respectively.
ThebasicstrengthofitwasdeterminedbyHammettindicator.
Calcinedhydrotalcitecontainedsurfacebasicsitesoflow(OHgroup),medium(Mg-Opairs)andstrong(O2)basicities.
ThemainbasicsiteswithHwereintherangeof7.
2e9.
8andtheothersiteswithHwereintherangeof9.
8e15.
ThesimilarresultswereobtainedbyXieetal.
[17,26].
3.
2.
TransestericationreactionBiodieselwasproducedbyatwo-stepprocess.
Therststep-pretreatment(acid-esterication)ofJatrophaoilwasstudiedinourpreviouswork[10],sointhispaperonlythesecondstepsolidbasecatalytictransestericationexperimentswereintroducedanddiscussed.
Fig.
3.
SEMimagesof(A)hydrotalcite,and(B)calcinedhydrotalciteat773Kfor6h.
Fig.
4.
AFMimagesofthecalcinedhydrotalciteat773Kfor6h.
X.
Dengetal.
/Energy36(2011)777e784780Thetransestericationprocessconsistedofthreeconsecutivereversiblereactions,whereinJatrophaoilwassuccessivelycon-vertedintodiglycerideandmonoglyceride,andnallyintoglycerinandFAMEs(fattyacidmethylesters)[26,27].
Fig.
5gavetheproducedbiodieselconsistingofsixFAMEs:palmiticacid(C16:0)13.
79%,palmitoleicacid(C16:1)0.
95%,stearicacid(C18:0)6.
33%,oleicacid(C18:1)42.
61%,linoleicacid(C18:2)26.
34%,andlinolenicacid(C18:3)0.
06%.
Themolarratioofmethanol/Jatropha-oilwasoneoftheimportantfactorsthataffectedtheyieldofmethylesters.
Stoi-chiometrically,3molofmethanolwererequiredforeachmoleofJatrophaoil(Eq.
(2)).
However,inpractice,methanol/oilmolarratioshouldbehigherthanthatofstoichiometryinordertodrivethereactiontowardcompletionandproducemoremethylesters.
Theeffectsofmethanol/Jatropha-oilmolarratioonbiodieselyieldweregiveninFig.
6a.
Whentheratioincreasedfrom3/1to4/1,theyieldofmethylestersroseconsiderablyfrom63.
0to94.
7%.
BecauseJatrophaoilwasimmisciblewithmethanol,thereactionwasincompleteandlimitedbydiffusionandthermodynamicprocess.
So,excessivemethanolshouldbeusedtopromotethereaction(Eq.
(2)).
Themaximumyieldof94.
7%wasachievedwhenthemolarratiowascloseto4/1.
Highermolarratiooverthestoichio-metricvalueresultedinahighrateofestersformationandcouldensurecompletereaction[28].
However,itwasobservedthatatmolarratiosabove4/1,excessivemethanolhadnosignicanteffectontheyield.
Conversely,alongertimewasrequiredforthesubsequentseparationstagebecauseseparationoftheesterslayerfromglycerolwasdifcultduetothefactthatmethanolwithonepolarhydroxylgroupcouldemulsifytheproducts[29].
Hence,thebestmethanol/oilmolarratiowasselectedas4/1.
Thecalcinednanoparticlesascatalystexhibitedhighactivitybecausetheypossessedstrongbasicsitesandalargesurfacearea.
Thetransestericationreactionwasstronglyaffectedbycatalystconcentration.
Withoutaddingcatalyst,littlereactionoccurred.
TheeffectofcatalystconcentrationonbiodieselyieldwasgiveninFig.
6b.
Whenthecatalystconcentrationincreasedfrom0.
5to1.
0wt%,estersyieldwasraisedfrom53.
8%tothemaximumyieldof93.
9%.
Thereasonwasthatcatalystconcentrationrosefrom0.
5to1.
0wt%couldincreasecontactbetweenreactantsandcatalyst.
However,whenthecatalystconcentrationincreasedfurtherabove1.
0wt%,biodieselyielddropped,whichwaspossiblyduetoamixingprobleminvolvingreactants,productsandsolidcatalyst.
Further-more,whenexcessivecatalystwasused,thetransestericationprocesswaseasilyemulsiedandresultedinhardseparationofproducts.
Sotheoptimizedcatalystconcentrationwas1.
0wt%.
Table1Physical-chemicalpropertiesofcalcinedhydrotalciteat773Kfor6h.
sampleMg/Almolarratio(actual)Surfacearea(m2/g)Porevolume(cm3/g)Porediameter(nm)Basicity(mmol/g)Mg-Al(3/1)2.
782180.
173.
93.
4Fig.
5.
GCgraphforbiodiesel.
Yield(%)502345660708090MethanoltooilmolarratioYield(%)Yield(%)ba50500.
250.
50.
751.
01.
256070809030831331832332860708090Temperature(K)Catalystconcentration(wt%)Yield(%)5060708090150180210240270Ultrasonicpower(W)cdFig.
6.
Effectsofvariablesonbiodieselyield:(a)methanoltooilmolarratio;(b)catalystconcentration;(c)temperature;and(d)ultrasonicpower.
X.
Dengetal.
/Energy36(2011)777e784781Reactiontemperaturewasalsoanimportantfactorthatinu-encedbiodieselyield.
Eachexperimentwasrunfor1.
5hwith1wt%catalystand4/1molarratioofmethanol/oil.
Theresults(Fig.
6c)indicatedthatbiodieselyieldwaslowatlowtemperatureswithonly52.
4%yieldat303Kfor1.
5h.
Biodieselyieldincreasedsharplyastemperaturerose,andreached94.
2%at318K.
However,theyielddecreasedastemperatureincreasedfurther.
Whentemperaturewashigherthan318K,methanolwouldvaporizeandformalotofbubbles,whichcouldinhibitreactionsonthethree-phaseinterface.
Therefore,theoptimizedtemperaturewasaround318K.
Effectofultrasonicpoweronbiodieselyieldwasinvestigatedwithmethanol/Jatropha-oilmolarratioof4/1,1wt%catalystandtemperatureof318K(Fig.
6d).
Biodieselyieldincreasedfrom77.
7%to94.
5%asultrasonicpowerrosefrom180to210W.
Inbiodieselproduction,Jatrophaoil,methanolandsolidcatalystformedathree-phasereactionmixtureduetotheirimmiscibility.
Ultra-sonicirradiationpromotedsufcientmixing,helpedtheformationandcollapseofmicro-scalebubblestogeneratelocalhightemperatureandhighpressure,andalsoprovidedalternativeenergysourcetopromotereactions[30].
However,astheultrasonicpowerincreasedfurtherfrom210to270W,biodieselyielddroppedfrom94.
5to76.
9%.
Thepossiblereasonwasthatmethanolwasvaporizedthatitwasseenfogformedontheliquidsurfaceathigherultrasonicpower.
Attheoptimizedcondition,i.
e.
,methanol/oilmolarratioof4/1,catalystconcentrationof1.
0wt%,reactiontemperatureof318K,andultrasonicpowerof210W,threerepeatedtestswerecon-ductedandanaveragebiodieselyieldof95.
20.
6%wasachievedin1.
5h.
Theyield(95.
2%)ishigherthanpreviousworksreported(e.
g.
,67%,90%)thatwereconductedatsevererreactionconditions(e.
g.
,9h,353e433K)[17,18].
Thepossiblereasonforthehighconversionrateisduetoultrasonicradiationthathelpsmixingreactantswithcatalystandpromotesreactions,andthehighlyactivenanocatalystssynthesizedbyusingureaasprecipitatorandwithMHT.
3.
3.
PropertiesofbiodieselOwingtothehighFFAs,withoutpretreatment,directtrans-estericationofJatrophaoilwaseasilyemulsiedandthebiodieselproducedwasnotstable.
Afterstoredfor1year,thebiodieselprecipitatedatthebottomofthecontainer.
Thetwo-stepprocesswassuccessfullyusedtoproducehighqualiedbiodiesel.
Itspropertiessuchasdensity,ashpoint,viscosity,AVandcetanenumber,wereclosetothoseoftheGermanstandard(DINV51606;Table2).
ThesimilarresultswereobtainedbyJainandSharma[31],DeOliveiraetal.
[32]andTiwarietal.
[33].
3.
4.
StabilityofbiodieselBiodieselwassynthesizedfromJatrophaoilbythetwo-stepprocess.
TheAV,viscosity,densityandchemicalcompositionofthefreshbiodieselandthestoredbiodieselfor1yearwereshowninTable3.
Afterstoredfor1year,theAV,viscosityanddensityincreasedslightly,from0.
154,3.
89and0.
886to0.
36,4.
05and0.
887,respectively.
However,theywerestillclosetothoseoftheGermanstandard(DINV51606;Table2).
Thechemicalcompositionindicatedthatbiodieselstoredfor1year,saturatedfattyacidsincreasedslightly,palmiticacidfrom13.
79%to15.
20%andstearicacidfrom6.
33%to8.
37%,respectively.
Unsaturatedfattyacidsweredecreased,palmitoleicacidfrom0.
98%to0.
87%,oleicacidfrom42.
61%to38.
96%andlinoleicacidfrom26.
34%to25.
41%,respec-tively.
Thepossiblereasonwasunsaturatedfattyacidswereunstableandoxidizedafterstoredforlongtime.
LinolenicacidwasnotdetectedbyGCafterstored1year.
Itcouldbeconcludedthatthebiodieselwasstableandqualiedforuse.
3.
5.
CatalystdeactivationDeactivationofsolidbasiccatalystforbiodieselproductionwascausedbythethreemainreasons.
Firstly,productsandby-prod-uctswereabsorbedonthesurfaceofthecatalystthatwoulddecreasethecontactbetweenbasicsitesandreactants.
Secondly,theactivitysiteswereleachedpartlyintosolutions.
Thelastreasonwasthatthecatalyststructurecollapsed.
Biodieselyieldwas95.
2%ifthecatalystwasrst-timeused.
Afterreaction,theusedcatalystwasseparatedbyhigh-speedcentrifugation,andreusedascatalystforthesecondtime.
Biodieselyieldwasdecreasedto90.
4%.
Forthethirdtimeused,biodieselyieldwasonly80.
7%.
Theservicelifeofthecalcinedhydrotalciteforbio-dieselproductionwasonly3times.
Afterthecatalystwassepa-rated,wefoundthatviscousliquidwasabsorbedonthesurfaceofthecatalyst.
FT-IR(FourierTransformInfrared)spectrum(Fig.
7)showedthattheliquidwasglycerolthatdeactivatedthecatalyst.
Therefore,theviscousliquidonthecatalystwasremovedbywashingwithethanol,andthecatalystwasreused.
Atthe8thtimereused,biodieselyieldwas89.
1%.
Butatthe9thtime,biodieselyieldsharplydecreasedtoabout43.
7%.
Thecatalystwasdeacti-vatedbutwithoutleachabilityofMg2andAl3intheliquidproductsasconrmedbyICP(InductivelyCoupledPlasma)[34].
SEMimageofthedeactivatedcatalystwasshowninFig.
8.
Thecatalysthadaplatelet-likestructure(402nmwidthand64nmthickness),butitwasnotasuniformastheinitialcalcinedhydrotalcite(Fig.
3B).
ItwasfoundthattheactivesitesofthedeactivatedcatalystdisappearedduetoitsstructurecollapsedidentiedbyXRD[35].
Table2PropertiesofJatrophabiodiesel.
ParameterJatrophaoilBiodieselTheGermanStandard(DINV51606:1997)Density(g/mL,289K)0.
8920.
8860.
875e0.
900Flashpoint(K)498459!
373Viscosity(mm2/s,313K)24.
53.
893.
5e5.
0Acidvalue(mgKOH/g)10.
50.
1540.
5Freemethanolcontent(wt%)e0.
20.
3Freeglycerolcontent(wt%)e0.
160.
2Totalglycerolcontent(wt%)e0.
180.
25Sulphurcontent(wt%)e0.
0030.
01Cetanenumber5158!
49Watercontent/(mg/kg)5200172300Ashcontent(wt%)e0.
0240.
05Pourpoint(K)271268eCarbonresidue(%)1.
00.
20.
05Copperstripcorrosion1a1a1aCaloricvalue(MJ/kg)38.
6541.
72eColoryellowbuffeTable3StabilityofJatrophabiodiesel.
ParameterBiodiesel(freshproduced)Biodiesel(storedfor1year)Density(g/mL,289K)0.
8860.
887Viscosity(mm2/s,313K)3.
894.
05Acidvalue(mgKOH/g)0.
1540.
36palmiticacid13.
79%15.
2%palmitoleicacid0.
95%0.
87%stearicacid6.
33%8.
37%oleicacid42.
61%38.
96%linoleicacid26.
34%25.
41%linolenicacid0.
06%eX.
Dengetal.
/Energy36(2011)777e7847824.
ConclusionSolidbasenanocatalystderivedfromhydrotalciteswithMg/Almolarratioof3/1wassynthesizedbycoprecipitationmethodusingureaasprecipitatorandwithMHT,followedbycalcination.
Thecatalystwasonly7.
3nmbycalculation,butaccordingtoAMFanalysis,theycongregatedtoformalayeredstructurewithsizeaslargeas0.
941-mmwidthand381-nmthickness.
Owingtoitsstrongbasicity,thecatalystwasusedforthetransestericationofJatrophaoilcon-taining5-12AVstobiodieselafterpretreatment.
Itwasfoundthatat210Wultrasonicpower,whenmethanolreactedwiththeoil(4/1molarratio)mixedwith1.
0wt%catalystat318Kfor1.
5h,biodieselyieldof95.
2%wasobtainedthatwashigherthanpreviousreported,thebiodieselpropertieswereclosetothoseoftheGermanstandard(DINV51606).
Themainreasonsforcatalystdeactivationwerethesurfaceabsorptionofby-productglycerolaswellasthecollapseofthelayeredstructure.
Afterremovingtheglycerolonthesurface,thecatalystwasreusedfor8times.
ItcouldbeconcludedthatthecalcinedhydrotalcitenanocatalystcombinedwithultrasonicradiationisaneffectivemethodfortheproductionofbiodieselfromJatrophaoil.
AcknowledgmentsTheauthorswishtoacknowledgethenancialsupportfromChineseAcademyofSciences[BairenJihuaandknowledgeinno-vationkeyproject(KSCX2-YW-G-075)],andChinaNationalScienceFoundation(No:21076220).
References[1]MaFR,HannaMA.
Biodieselproduction:areview.
BioresourceTechnology1999;70:1e15.
[2]PramanikK.
PropertiesanduseofJatrophacurcasoilanddieselfuelblendsincompressionignitionengine.
RenewableEnergy2003;28:239e48.
[3]DemirbasA.
Progressandrecenttrendsinbiodieselfuels.
EnergyConversionandManagement2009;50:14e34.
[4]MacorA,PavanelloP.
Performanceandemissionsofbiodieselinaboilerforresidentialheating.
Energy2009;34:2025e32.
[5]FernandoSD,KarraP,HernandezR,JhaSK.
Effectofincompletelyconvertedsoybeanoilonbiodieselquality.
Energy2007;32:844e51.
[6]ChenKS,LinYC,HsiehLT,LinLF,WuCC.
Savingenergyandreducingpollutionbyuseofemulsiedpalmbiodieselblendswithbio-solutionadditive.
Energy2010;35:2043e8.
[7]GuiMM,LeeKT,BhatiaS.
Feasibilityofedibleoilvs.
non-edibleoilvs.
wasteedibleoilasbiodieselfeedstock.
Energy2008;33:1646e53.
[8]GaoYY,ChenWW,LeiH,LiuY,LinX,RuanRS.
Optimizationoftrans-estericationconditionsfortheproductionoffattyacidmethylester(FAME)fromChinesetallowkerneloilwithsurfactant-coatedlipase.
BiomassBio-energy2008;33:277e82.
[9]YangCY,DengX,FangZ,PengDP.
SelectionofhighoilyieldseedsourcesofJatrophacurcasL.
forbiodieselproduction.
Biofuels2010;1:705e17.
[10]DengX,FangZ,LiuYH.
UltrasonictransestericationofJatrophacurcasL.
oiltobiodieselbyatwo-stepprocess.
EnergyConversionManagement2010;51:2802e7.
[11]OpenshawK.
AreviewofJatrophacurcas:anoilplantofunfullledpromise.
BiomassBioenergy2000;19:1e15.
[12]TamlampudiS,TalukderMR,HamaS,NumataT,KondoA,FukudaH.
Enzy-maticproductionofbiodieselfromJatrophaoil:acomparativestudyofimmobilizedwholecellandcommerciallipasesasbiocatalyst.
BiochemistryEngineeringJournal2007;39:185e9.
[13]AntolínG,TinautFV,BriceoY,CastaoV,PérezC,RamírezAI.
Optimizationofbiodieselproductionbysunoweroiltransesterication.
BioresourceTechnology2002;83:111e4.
[14]TanKT,LeeKT,MohamedAR.
Potentialofwastepalmcookingoilforcatalyst-freebiodieselproduction.
Energy2010;35:1e4.
[15]ZengHY,FengZ,DengX,LiYQ.
ActivationofMg-Alhydrotalcitecatalystsfortransestericationofrapeoil.
Fuel2008;87:3071e6.
[16]TantirungrotechaiJ,ChotmongkolsapP,PohmakotrM.
Synthesis,character-ization,andactivityintransestericationofmesoporousMg-Almixedmetaloxides.
MicroporousandMesoporousMaterials2010;128:41e7.
[17]XieWL,PengH,ChenLG.
CalcinedMg-Alhydrotalcitesassolidbasecatalystsformethanolysisofsoybeanoil.
JournalMolecularCatalysisA:Chemistry2006;246:24e32.
[18]BritoA,BorgesME,GarínM,HernándezA.
BiodieselproductionfromwasteoilusingMg-Allayereddoublehydrotalcitecatalysts.
Energy&Fuels2009;23:2952e8.
[19]DengX.
MicrostructurecontrollablepreparationandapplicationofNano-crystallineMg-Alhydrotalcite,MSc.
Thesis(inChinese),DepartmentofChemicalEngineering,XiangtanUniversity,China;2007,p.
77e86.
[20]FraileJM,GarcíaN,MayoralJA.
TheinuenceofalkalinemetalsonthestrongbasicityofMg-Almixedoxides:thecaseoftransestericationreactions.
AppliedCatalysisA:General2009;364:87e94.
[21]FangF.
Thepreparationofbiodieselandthestudiesonits'analyticalmethodofqualityindex,MSc.
Thesis(inChinese),DepartmentofChemicalEngi-neering,XiangtanUniversity,China;2005,p.
44e60.
[22]AramendíaMA,BorauV,JiménezC,MarinasJM,RuizJR,UrbanoFJ.
Catalytichydrogentransferfrom2-propanoltocyclohexanoneoverbasicMg-Aloxides.
AppliedCatalysisA:General2003;255:301e8.
[23]BirksLS,FriedmanH.
ParticlesizedeterminationfromX-raylinebroadening.
JournalofAppliedPhysics1946;17:687e91.
[24]FangZ,AssaaoudiH,GuthrieRIL,KozinskiJA,ButlerIS.
Continuoussynthesisoftinandindiumoxidenanoparticlesinsub-andsupercriticalwater.
JournalAmericanCeramicSociety2007;90:2367e71.
[25]KusˇtriwskiP,ChmielarzE,BozekE,SawalhaM,RoessnerF.
AcidityandbasicityofhydrotalcitederivedmixedMg-AloxidesstudiedbytestreactionofMBOHconversionandtemperatureprogrammedde-sorptionofNH3andCO2.
JournalMaterialsResearchBulletin2004;39:263e81.
[26]XieWL,LiH.
Alumina-supportedpotassiumiodideasaheterogeneouscata-lystforbiodieselproductionfromsoybeanoil.
JournalMolecularCatalysisA:Chemistry2006;255:1e9.
[27]BarakosN,PasiasS,PapayannakosN.
TransestericationoftriglyceridesinhighandlowqualityoilfeedsoveranHT2hydrotalcitecatalyst.
BioresourceTechnology2008;99:5037e42.
Fig.
8.
SEMimageofthedeactivatedcatalyst.
Fig.
7.
FT-IRspectrumoftheviscousliquidonthesurfaceoftheusedcatalyst.
X.
Dengetal.
/Energy36(2011)777e784783[28]LeungDY,GuoY.
Transestericationofneatandusedfryingoil:optimizationforbiodieselproduction.
FuelProcessingTechnology2006;87:83e90.
[29]LiuXH,XiongXY,LiuCM,LiuDY,WuAJ,HuQL,etal.
PreparationofbiodieselbytransestericationofrapeseedoilwithmethanolusingsolidbasecatalystcalcinedK2CO3/g-Al2O3.
JournalofAmericanOilChemistsSociety2010;87:817e23.
[30]HinguSM,GogatePR,RathodVK.
Synthesisofbiodieselfromwastecookingoilusingsonochemicalreactors.
UltrasonicsSonochemistry2010;17:827e32.
[31]JainS,SharmaMP.
ProspectsofbiodieselfromJatrophainIndia:areview.
RenewableandSustainableEnergyReviews2010;14:763e71.
[32]DeOliveiraJS,LeitePM,DeSouzaLB,MelloVM,SilvaEC,RubimJC,etal.
CharacteristicsandcompositionofJatrophagossypiifoliaandJatrophacurcasL.
oilsandapplicationforbiodiesel.
BiomassandBioenergy2009;33:449e53.
[33]TiwariAK,KumarA,RahemanH.
BiodieselproductionfromJatrophaoil(Jatrophacurcas)withhighfreefattyacids:anoptimizedprocess.
BiomassandBioenergy2007;31:569e75.
[34]LiuXJ,PiaoXL,WangYJ,ZhuSL,HeHY.
Calciummethoxideasasolidbasecatalystforthetransestericationofsoybeanoiltobiodieselwithmethanol.
Fuel2008;87:1076e82.
[35]GaoLJ,TengGY,LvJH,XiaoGM.
BiodieselsynthesiscatalyzedbytheKF/Ca-Mg-Alhydrotalcitebasecatalyst.
EnergyFuels2010;24:646e51.
X.
Dengetal.
/Energy36(2011)777e784784
Theseparticlesweremicro-sizedmixedMg/AloxidesascharacterizedbySEMandAFM.
ButactuallytheywerenanosizedaccordingtothecalculationsfromXRDdata.
Becauseoftheirstrongbasicity,thenanoparticleswerefurtherusedascatalystforbiodieselproductionfromJatrophaoilafterpretreatment.
Experimentswereconductedwiththesolidbasiccatalystinanultrasonicreactorunderdifferentconditions.
Attheoptimizedcondition,biodieselyieldof95.
2%wasachieved,andthebiodieselpropertieswereclosetothoseoftheGermanstandard.
Thecatalystcanbereusedfor8times.
2010ElsevierLtd.
Allrightsreserved.
1.
IntroductionBiodieselhasdrawnmoreandmoreattentioninrecentyearsbecauseitisrenewableandhaslessdetrimentaleffectsonenvi-ronmentascomparedwithconventionaldieselderivedfrompetroleum[1].
Biodieselobtainedfromrenewablebiomassfeed-stockscanbeusedindieselenginesorblendedatvariousproportionswithpetroleumdieselasfuel[2].
Itconsistsofmono-alkylestersthatareusuallyproducedbytransestericationofplantoilwithmethanolorethanol.
Ithassimilarandsometimesbetterphysicalandchemicalpropertiesthanpetroleumdiesel,suchashigherashpoint,ultra-lowersulphurconcentration,betterlubricatingefciencyandfewpollutantsproduced[3e6].
However,biodieselisexpensiveduetothehighpriceofplantoilandsomeprocessingtechnologicalissues,suchascatalystandequipment.
Therefore,littlecommercialbiodieselisusedinChina.
Therawmaterialsexploitedcommerciallybysomedevelopedcountriesareedibleoilssuchas,rapeseed,soybean,palm,sunower,coconutandlinseedoils[3,7].
Thefractionofrawmaterialsforworldcommercialbiodieselproductionisrapeseedoil84%,sunoweroil13%,palmoil1%,soybeanoilandothers2%[7].
UsingedibleoilstoproducebiodieselindevelopingcountriessuchasChinawithlimitedarablelandpercapitaisnotfeasibleandisbanned.
Therefore,onlynon-edibleplantsareconsideredasfavorableresourcesforbiodieselproduction,suchasChinesetallow[8]andJatrophacurcasL.
trees[9,10],whichareproducingnon-edibleoilinappreciablequantityandcanbegrowninalarge-scaleonnon-croppedmarginallandsandwastelands.
JatrophacurcasL.
,adrought-resistantshrubortree,iswidelydistributedinthewildinsemi-cultivatedtropicalorsubtropicalareasinCentralandSouthAmerica,Africa,India,andSouthChina.
Ithasaproductivelifespaninexcessof30years.
ThefattyacidcompositionofJatrophaoilissimilartootheredibleoils,butthepresenceofsometoxicmaterialsinkernel(e.
g.
,curcin)renderstheoilunsuitableforcookingpurposes[11,12].
TheoilcontentinJatrophaseedrangesfrom25%to40%byweightandinthekernelitselfrangesfrom45%to60%[10].
Nowadays,Jatrophatrees,asapotentialalternativebiodieselcrop,arewidelycultivatedinSouthwestofChinasuchasYunnan,Sichuan,andGuangxiprovinces[9].
Inthenearfuture,itwillsupplypartofcrudeoilforcommercialbiodieselproductioninChina.
TheconventionalindustrialproductionofbiodieselisthroughtransestericationofcrudeoilwithahomogeneousstrongbasecatalystsuchasNaOHandKOH[1,3,13].
Afterreaction,recoveryofglycerol,removalofbasecatalystfromproducts,andtreatmentofalkalinewastewaterarecostlyandnon-environmental.
Further-more,ahomogeneousbasecatalystisineffectiveforproductionbiodieselfromhighacid-valuecrudeoilsduetotheformationofsoap.
Tanetal.
[14]reportedacatalyst-freebiodieselproductionmethod,usingwastepalmcookingoilasrawmaterialand*Correspondingauthor.
Tel.
:868715163360;fax:868715160916.
E-mailaddress:zhenfang@xtbg.
ac.
cn(Z.
Fang).
URL:http://brg.
groups.
xtbg.
cn/ContentslistsavailableatScienceDirectEnergyjournalhomepage:www.
elsevier.
com/locate/energy0360-5442/$eseefrontmatter2010ElsevierLtd.
Allrightsreserved.
doi:10.
1016/j.
energy.
2010.
12.
043Energy36(2011)777e784supercriticalmethanol.
Butthemethodhasahighcostinreactorandoperation(duetohighpressuresandhightemperatures),andhighmethanolconsumption(e.
g.
,highmethanol/crude-oilmolarratioof40/1).
Therefore,heterogeneoussolidcatalystswereusedforthetransestericationprocess,whereabetterseparationandreuseofthecatalystswithoutsaponicationwereachieved[15].
Preparationandapplicationofsolidsuperbasecatalystsareanemergingareathatisattractingmoreandmoreattention,becausethecatalystsareeasilyseparatedforreuseandpossessahighactivityforvariousreactionsundermildconditions.
Theycanreplacehomogeneousbasecatalystsinordertominimizetheproductionofpollutants.
LDHs(Layereddoublehydroxides)areimportantinorganicmaterialswithlayeredstructureandanionexchangedcapacity.
Asclassicalsolidbasematerials,calcinedLDHsarewidelyusedascatalystintheproductionofbiodiesel[16e18].
Inpreviouswork[17],transestericationprocesswascarriedoutwithreuxofmethanol,methanol/soybean-oilmolarratioof15/1,reactiontimeof9handcatalystamountof7.
5%,andoilconversionratewasonly67%.
IntheworkofBritoetal.
[18],wasteoilasfeedstock,biodieselproductionwasperformedathightempera-turesrangingfrom353to433K,methanol/oilmolarratiofrom12/1to48/1andcatalystconcentrationfrom3to12%,respectively,and90%biodieselyieldwasachieved.
Ultrasonicradiationisarelativenewtechniquethatresultsintheformationandcollapseofmicro-scalebubblesinliquidtogeneratelocalhightemperatureandhighpressure.
So,itcanbeusedasanalternativeenergysourcetopromotereactions.
Itenormouslyreducesreactiontime,methanol/oilmolarratioandcatalystconcentration.
Therefore,biodieselproductionwithcalcinedhydrotalcitecatalystcoupledwithultra-sonicradiationislikelytobecomeaneweffectivemethod.
Thepurposeofthisworkistosynthesizehydrotalciteparticlesandsubsequentlytocalcinethemascatalystforbiodieselproduc-tion.
HydrotalciteparticleswerepreparedinanultrasonicreactorbycoprecipitationofaqueousnitratesolutionsofMgandAlusingureaasprecipitatingagent,andsubsequentlywithMHT(micro-wave-hydrothermaltreatment).
Thesynthesizedparticles,aftercalcination,wereusedassolidbasiccatalystinthetrans-estericationofJatrophaoiltotesttheiractivitiesintheultrasonicreactor.
Experimentsunderdifferentreactionconditions,suchasmethanol/Jatropha-oilmolarratio,catalystconcentration,reactiontemperatureandultrasonicpower,wereperformedinordertooptimizebiodieselyield.
Thereasonsforcatalystdeactivationwerealsostudied.
2.
Experimental2.
1.
MaterialsNitratesofMgandAl(99.
8%purity)usedinthesynthesiswereobtainedfromChengdouFineChemicalCo.
Ltd.
Urea(99.
0%purity)andmethanol(99.
0%purity)werepurchasedfromShanghaiFineChemicalCo.
Ltd.
JatrophaoilwithhighFFAs(free-fatty-acids)wasobtainedfromourinstituteinXishuangbanna,Yunnanprovince,SouthwestofChina[9].
AccordingtotheChineseNationalstan-dardsGB9014.
2-88andGB164-64,AV(acidvalue)andSV(saponicationvalue)oftheJatrophaoilweremeasuredas10.
5mgKOH/gand191.
0mgKOH/g,respectively.
Soitsmolecularweightwascalculatedas932g/molbytheformula:M(56.
110003)/(SVAV)[19].
2.
2.
CatalystpreparationHydrotalciteparticleswithMg/Almolarratioof3/1weresynthesizedbyacoprecipitationmethodusingureaasprecipitatingagent,andwithMHT.
Inthismethod,asolution(0.
15MMgnitrateand0.
05MAlnitrate)wasmadeinathree-neckaskbydissolvingthetwosolidnitratesindeionizedwater.
Urea{[urea]/[NO3]molarratioof4/1}wasthenaddedintotheask.
Theaskwassubmergedinanoilbathat373Kwithstirringabout500rpmintheultrasonicreactorfor2h.
TheslurryobtainedwassubsequentlysubmittedtoMHTinthemotherliquorfor2hat393K.
Afterltered,washedthoroughlywithdeionizedwateranddriedat373Kfor10hatavacuumcondition,solidhydrotalciteparticleswereobtained.
TheywerecharacterizedbypowderXRD(X-RayDiffraction).
Catalyticactivityofhydrotalcitewasstudiedbythetrans-estericationofJatrophaoilwithmethanoltobiodiesel.
Withoutcalcination,hydrotalcitedisplayednoparticularcatalyticactivityinthereaction.
But,aftercalcined,hydrotalcitewasintheformofOHcontainedmixedAl-Mgoxideswhichhadheterogeneoussurfacebasicitywithbasicsitesoflow(OH-groups),medium(Mg-Opairs),andstrong(O2anions)basicityandexhibitedasignicantactivity[20].
So,hydrotalciteparticleswerecalcinedat773Kfor6hinamufefurnaceandusedascatalystforthefollowingbiodieselproduction.
2.
3.
BiodieselproductionBiodieselproductionprocessisstronglyinuencedbythefattyacidcompositionandFFAscontentofcrudeoil.
TheAVoffreshJatrophaoilinXishuangbanna,Yunnanprovince,isfrom5to12mgKOH/g.
OwingtoitshighFFAs,thetransestericationofJatrophaoiltobiodieselcatalyzeddirectlybysolidcatalysthadalowconver-sionrate.
Evenusinglongerreactiontime,only80.
4%biodieselyieldwasachieved.
Inordertoincreasebiodieselyield,FFAsinJatrophaoilneedtoremove.
Atwo-stepprocessofJatrophaoiltobiodieselwasusedtoresolvetheproblem[10].
First,anacid-estericationpretreatmentwasperformedwithconcentratedsulfuricacidascatalyst.
Thethree-neckaskwithawater-cooledcondenserwaslledwith200-mLJatrophaoil,40-mLanhydrousmethanoland4-mLsulfuricacid.
Themixturewasvigorouslystirredandreuxedat318Kinanultrasonicreactorfor1.
5h.
Afterreaction,themixturewasltered,andtheunreactedmethanolwasseparatedfromtheliquidphaseviarotaryevapo-ration.
Theoilwaswashedthreetimeswithsodiumchloridesolutionthendriedusinganhydroussodiumsulfate.
Afterthepretreatment,theAVofthepretreatedoilwasreducedtoonly0.
7mgKOH/gwith93.
3%estericationrate.
Thereactionequationwasdescribedasbelow:RCOOHCH3OH/RCOOCH3H2O(1)Thesecondstep,abase-catalyzedtransestericationreaction(Eq.
(2))wascarriedoutinthesameultrasonicreactorwiththecalcinedhydrotalcitecatalyst:CH2OOCR1jCHOOCR2jCH2OOCR33CH3OH#CatalystCH2OHjCHOHjCH2OHR1COOCH3jR2COOCH3jR3COOCH3(2)Biodieselyieldwascalculatedas:Biodieselyield%totalactualweightofmethylesters100=totaltheoreticalweightofmethylesters3where,totalweightofmethylesterswereproducedfrombothsteps,totaltheoreticalweightwasobtainedassuming100%conversionoffattyacidsinJatrophaoilaccordingtoEqs.
1and2.
Thereactionprocedurewasasfollows:rst,thecatalystwasdispersedinmethanolwithmechanicalstirring(about600rpm).
X.
Dengetal.
/Energy36(2011)777e784778Then,theabovepretreatedoilwasaddedintothemixtureandheatedto318Kbywaterbath.
Afterreaction,excessivemethanolwasdistilledoffunderavacuumconditionwhilecatalystwasseparatedbyhigh-speedcentrifugation.
Afterremovaloftheglyc-erollayer,biodieselwascollectedandreadyforChromatographicanalyses.
2.
4.
Jatrophaoilandbiodieselanalyses2.
4.
1.
JatrophaoilThecompositionofcrudeoilwasanalyzedbyGasChromatog-raphy(GC,Shimadzu,GC-2014)withaameionizationdetectorandacapillarycolumn(Rtx-Wax30m0.
25mm0.
25mm).
Oxygen-freenitrogenwasusedasacarriergasataowrateof1.
4mL/min.
Otherconditionswereasfollows:initialoventemperatureof443K(2min),rampat5.
0K/min,naltemperatureof503K(4min),injectortemperatureof523K,detectortemper-atureof553K,andasplitratioof39/1.
Theanalysiswascarriedoutbyinjecting1-mLsamplesolution(0.
25-mLcrudeJatropha-oildis-solvedin9.
75-mLn-hexane)intotheGC.
Undecanoicacidmethylesterwasusedastheinternalstandard.
ThefattyacidsinJatrophaoilwereidentiedandquantiedbycomparingtheirretentiontimesandpeakareastothoseofstandardfattyacids.
Fig.
1showssixfattyacidswereidentiedandquantiedas:palmiticacid(C16:0)15.
18%,palmitoleicacid(C16:1)0.
99%,stearicacid(C18:0)6.
25%,oleicacid(C18:1)41.
17%,linoleicacid(C18:2)31.
25%,andlinolenicacid(C18:3)0.
08%.
2.
4.
2.
BiodieselBiodieselwasalsoanalyzedbytheaboveGCmethodundersimilarconditions.
Thepreparedbiodiesel(5mL)wasdissolvedin20-mLdichloromethaneand1-mLinternalstandardsolutionforGCanalysis.
Samplesolutions(1mL)wereinjectedbyasampleratanoventemperatureof493K.
Heliumwasusedascarriergasataowrateof0.
8mL/min.
Identicationofmethylesterpeakswasdonebycomparingtheretentiontimesbetweenthesamplesandthestandardcompounds.
Methylesters(biodiesel)werequantiedbycomparingthepeakareasbetweenthesamplesandthestan-dardcompounds.
2.
5.
CharacterizationofhydrotalciteparticlesXRDanalyseswereconductedonaSiemensD-5000diffrac-tometerwithaCuKaradiationsourceatvoltageof40kVandcurrentof30mA,Ni-lteredandagraphitesecondarymono-chromater.
Sampleswereanalyzedinacontinuousscanmodebetween5and70(2q).
ScanningElectronMicroscope(SEM,HitachiS-520)measurementswerecarriedoutat11kV.
AFM(AtomicForceMicroscope)imageswereobtainedusingWET-SPM-9500-J3instrument(ShimadzuCo.
Ltd.
).
SamplesforAFMobser-vationweredispersedinanalcoholsolutionfor8hinanultrasonicreactor.
NitrogenphysisorptionwasconductedonaMicromeriticsASAP-2020surfaceareaandporosimetrysystem.
TheelementalanalysesweredeterminedbywavelengthdispersiveXRF(X-RayFluorescence)spectrometer(BrukerAXS).
2.
6.
PhysicochemicalpropertiesofbiodieselFlashpoint,pourpoint,viscosity,AVandcetanenumberofbiodieselweremeasuredaccordingtotheChinesenationalstan-dardsGB267-64,GB265-64,GB164-64andGB386-64.
Otherphysicochemicalpropertiesofbiodieselwereobtainedaccordingtoreferences[19]and[21].
3.
Resultsanddiscussion3.
1.
CharacterizationofhydrotalciteandcalcinedhydrotalciteXRDpatternsofhydrotalciteparticleswithMg/Almolarratioof3/1weregiveninFig.
2.
InFig.
2a,theparticlesexhibitedasinglephase,correspondingtoatypicalhydrotalcitestructure(JCPDSle70-2151)withstrong,sharp,andsymmetricpeaksforthe(003),(006),(110),and(113)planesaswellasbroadandsymmetricpeaksforthe(009),(015),and(018)planes[22].
Theaverageparticlesizewascalculatedas7.
3nmbytheSchererequation[Dck((l/b)cosq);whereDcistheaverageparticlesize;kistheSchererconstant(0.
89);listheX-raywavelength,(CuKa)0.
1541nm;bistheFWHM(full-widthathalf-maximum);qisthediffractionangle]intheXRD(003)reection[23,24].
Aftercalcinedat773Kfor6h,hydrotalciteparticlesweredecomposedtomixedMg-Alcomplexoxides,whichwereconrmedbytheXRDpatterninFig.
2b.
Forthecalcinedparticles,thecharacteristicreectionswereobservedclearlyat2qof43and63,correspondingtoanMgO-likephaseormagnesia-aluminasolidphase.
ThepeaksofAl2O3phasewereverysmall,indicatingthatAl3cationsweredispersedinthestructureofMgOwithouttheformationofspinelspecies[15,25].
SEMimagesofhydrotalciteandcalcinedhydrotalciteweregiveninFig.
3.
HydrotalcitehadarelativelyuniformhexagonalFig.
1.
GCgraphforcrudeJatrophaoil.
Fig.
2.
XRDpatternsofhydrotalcite:(a)withoutcalcination,and(b)aftercalcinedat773Kfor6h.
X.
Dengetal.
/Energy36(2011)777e784779platelet-likestructure,butpartlydestroyedatthecalcinedtemperatureof773K.
Beforecalcination,hydrotalcitehadawell-developedplateletstructureofatypicallayeredmaterialwithauniformsizeof1mmwidthand72nmthickness(Fig.
3A)thatismuchlargerthantheabovecalculatedvalue(7.
3nm).
Thepossiblereasonisthattheplateletswereformedbyagglomerationwithnumerousnenanoparticles.
Aftercalcinedat773K,theuniformhexagonalplateletstructurewaspartlydestroyedduetotheremovalofhydroxylgroupsinthemetalhydroxidelayersandcarbondioxidesproductionfromthedecompositionofCO32existedintheinterlayerspaceascharge-balancinganion.
Butitstillkeptsheetstructurewithasmallersizeof372nmwidthand72nmthickness(Fig.
3B).
AFMimagesofthecalcinedhydrotalciteparticlesat773Kfor6hwiththesolidstructure,diameterandthicknessweregiveninFig.
4.
Theparticlesofthemetalhydroxidelayerswerecongregatedtoformalargelayeredstructurewiththesizeof941nmwidthand381nmthickness.
However,theaverageparticlesizewasonly372nmwidthand72nmthicknessinFig.
3B,whichindicatedthatlargeparticleswereformedbyagglomerationthatwerehardtoseparateforAFManalysesevenafterultrasonicdispersiontreatment.
TheMg/AlatomicratiointhemixedmetaloxidesamplewasveriedbyXRFspectroscopy.
TheactualMg/Alatomicratiowas2.
78/1,thatiscloseto3/1ofthestartingMgandAlnitrates.
Phys-ical-chemicalpropertiesofcalcinedhydrotalciteweregiveninTable1,andtheyagreedwellwiththeearliermeasurementsbyTantir-ungrotechaietal.
[16].
TheMg-Almixedmetaloxidesampleexhibitedhighsurfaceareaof218m2/gwithporevolumeandporediameterof0.
17cm3/gand3.
9nm,respectively.
ThebasicstrengthofitwasdeterminedbyHammettindicator.
Calcinedhydrotalcitecontainedsurfacebasicsitesoflow(OHgroup),medium(Mg-Opairs)andstrong(O2)basicities.
ThemainbasicsiteswithHwereintherangeof7.
2e9.
8andtheothersiteswithHwereintherangeof9.
8e15.
ThesimilarresultswereobtainedbyXieetal.
[17,26].
3.
2.
TransestericationreactionBiodieselwasproducedbyatwo-stepprocess.
Therststep-pretreatment(acid-esterication)ofJatrophaoilwasstudiedinourpreviouswork[10],sointhispaperonlythesecondstepsolidbasecatalytictransestericationexperimentswereintroducedanddiscussed.
Fig.
3.
SEMimagesof(A)hydrotalcite,and(B)calcinedhydrotalciteat773Kfor6h.
Fig.
4.
AFMimagesofthecalcinedhydrotalciteat773Kfor6h.
X.
Dengetal.
/Energy36(2011)777e784780Thetransestericationprocessconsistedofthreeconsecutivereversiblereactions,whereinJatrophaoilwassuccessivelycon-vertedintodiglycerideandmonoglyceride,andnallyintoglycerinandFAMEs(fattyacidmethylesters)[26,27].
Fig.
5gavetheproducedbiodieselconsistingofsixFAMEs:palmiticacid(C16:0)13.
79%,palmitoleicacid(C16:1)0.
95%,stearicacid(C18:0)6.
33%,oleicacid(C18:1)42.
61%,linoleicacid(C18:2)26.
34%,andlinolenicacid(C18:3)0.
06%.
Themolarratioofmethanol/Jatropha-oilwasoneoftheimportantfactorsthataffectedtheyieldofmethylesters.
Stoi-chiometrically,3molofmethanolwererequiredforeachmoleofJatrophaoil(Eq.
(2)).
However,inpractice,methanol/oilmolarratioshouldbehigherthanthatofstoichiometryinordertodrivethereactiontowardcompletionandproducemoremethylesters.
Theeffectsofmethanol/Jatropha-oilmolarratioonbiodieselyieldweregiveninFig.
6a.
Whentheratioincreasedfrom3/1to4/1,theyieldofmethylestersroseconsiderablyfrom63.
0to94.
7%.
BecauseJatrophaoilwasimmisciblewithmethanol,thereactionwasincompleteandlimitedbydiffusionandthermodynamicprocess.
So,excessivemethanolshouldbeusedtopromotethereaction(Eq.
(2)).
Themaximumyieldof94.
7%wasachievedwhenthemolarratiowascloseto4/1.
Highermolarratiooverthestoichio-metricvalueresultedinahighrateofestersformationandcouldensurecompletereaction[28].
However,itwasobservedthatatmolarratiosabove4/1,excessivemethanolhadnosignicanteffectontheyield.
Conversely,alongertimewasrequiredforthesubsequentseparationstagebecauseseparationoftheesterslayerfromglycerolwasdifcultduetothefactthatmethanolwithonepolarhydroxylgroupcouldemulsifytheproducts[29].
Hence,thebestmethanol/oilmolarratiowasselectedas4/1.
Thecalcinednanoparticlesascatalystexhibitedhighactivitybecausetheypossessedstrongbasicsitesandalargesurfacearea.
Thetransestericationreactionwasstronglyaffectedbycatalystconcentration.
Withoutaddingcatalyst,littlereactionoccurred.
TheeffectofcatalystconcentrationonbiodieselyieldwasgiveninFig.
6b.
Whenthecatalystconcentrationincreasedfrom0.
5to1.
0wt%,estersyieldwasraisedfrom53.
8%tothemaximumyieldof93.
9%.
Thereasonwasthatcatalystconcentrationrosefrom0.
5to1.
0wt%couldincreasecontactbetweenreactantsandcatalyst.
However,whenthecatalystconcentrationincreasedfurtherabove1.
0wt%,biodieselyielddropped,whichwaspossiblyduetoamixingprobleminvolvingreactants,productsandsolidcatalyst.
Further-more,whenexcessivecatalystwasused,thetransestericationprocesswaseasilyemulsiedandresultedinhardseparationofproducts.
Sotheoptimizedcatalystconcentrationwas1.
0wt%.
Table1Physical-chemicalpropertiesofcalcinedhydrotalciteat773Kfor6h.
sampleMg/Almolarratio(actual)Surfacearea(m2/g)Porevolume(cm3/g)Porediameter(nm)Basicity(mmol/g)Mg-Al(3/1)2.
782180.
173.
93.
4Fig.
5.
GCgraphforbiodiesel.
Yield(%)502345660708090MethanoltooilmolarratioYield(%)Yield(%)ba50500.
250.
50.
751.
01.
256070809030831331832332860708090Temperature(K)Catalystconcentration(wt%)Yield(%)5060708090150180210240270Ultrasonicpower(W)cdFig.
6.
Effectsofvariablesonbiodieselyield:(a)methanoltooilmolarratio;(b)catalystconcentration;(c)temperature;and(d)ultrasonicpower.
X.
Dengetal.
/Energy36(2011)777e784781Reactiontemperaturewasalsoanimportantfactorthatinu-encedbiodieselyield.
Eachexperimentwasrunfor1.
5hwith1wt%catalystand4/1molarratioofmethanol/oil.
Theresults(Fig.
6c)indicatedthatbiodieselyieldwaslowatlowtemperatureswithonly52.
4%yieldat303Kfor1.
5h.
Biodieselyieldincreasedsharplyastemperaturerose,andreached94.
2%at318K.
However,theyielddecreasedastemperatureincreasedfurther.
Whentemperaturewashigherthan318K,methanolwouldvaporizeandformalotofbubbles,whichcouldinhibitreactionsonthethree-phaseinterface.
Therefore,theoptimizedtemperaturewasaround318K.
Effectofultrasonicpoweronbiodieselyieldwasinvestigatedwithmethanol/Jatropha-oilmolarratioof4/1,1wt%catalystandtemperatureof318K(Fig.
6d).
Biodieselyieldincreasedfrom77.
7%to94.
5%asultrasonicpowerrosefrom180to210W.
Inbiodieselproduction,Jatrophaoil,methanolandsolidcatalystformedathree-phasereactionmixtureduetotheirimmiscibility.
Ultra-sonicirradiationpromotedsufcientmixing,helpedtheformationandcollapseofmicro-scalebubblestogeneratelocalhightemperatureandhighpressure,andalsoprovidedalternativeenergysourcetopromotereactions[30].
However,astheultrasonicpowerincreasedfurtherfrom210to270W,biodieselyielddroppedfrom94.
5to76.
9%.
Thepossiblereasonwasthatmethanolwasvaporizedthatitwasseenfogformedontheliquidsurfaceathigherultrasonicpower.
Attheoptimizedcondition,i.
e.
,methanol/oilmolarratioof4/1,catalystconcentrationof1.
0wt%,reactiontemperatureof318K,andultrasonicpowerof210W,threerepeatedtestswerecon-ductedandanaveragebiodieselyieldof95.
20.
6%wasachievedin1.
5h.
Theyield(95.
2%)ishigherthanpreviousworksreported(e.
g.
,67%,90%)thatwereconductedatsevererreactionconditions(e.
g.
,9h,353e433K)[17,18].
Thepossiblereasonforthehighconversionrateisduetoultrasonicradiationthathelpsmixingreactantswithcatalystandpromotesreactions,andthehighlyactivenanocatalystssynthesizedbyusingureaasprecipitatorandwithMHT.
3.
3.
PropertiesofbiodieselOwingtothehighFFAs,withoutpretreatment,directtrans-estericationofJatrophaoilwaseasilyemulsiedandthebiodieselproducedwasnotstable.
Afterstoredfor1year,thebiodieselprecipitatedatthebottomofthecontainer.
Thetwo-stepprocesswassuccessfullyusedtoproducehighqualiedbiodiesel.
Itspropertiessuchasdensity,ashpoint,viscosity,AVandcetanenumber,wereclosetothoseoftheGermanstandard(DINV51606;Table2).
ThesimilarresultswereobtainedbyJainandSharma[31],DeOliveiraetal.
[32]andTiwarietal.
[33].
3.
4.
StabilityofbiodieselBiodieselwassynthesizedfromJatrophaoilbythetwo-stepprocess.
TheAV,viscosity,densityandchemicalcompositionofthefreshbiodieselandthestoredbiodieselfor1yearwereshowninTable3.
Afterstoredfor1year,theAV,viscosityanddensityincreasedslightly,from0.
154,3.
89and0.
886to0.
36,4.
05and0.
887,respectively.
However,theywerestillclosetothoseoftheGermanstandard(DINV51606;Table2).
Thechemicalcompositionindicatedthatbiodieselstoredfor1year,saturatedfattyacidsincreasedslightly,palmiticacidfrom13.
79%to15.
20%andstearicacidfrom6.
33%to8.
37%,respectively.
Unsaturatedfattyacidsweredecreased,palmitoleicacidfrom0.
98%to0.
87%,oleicacidfrom42.
61%to38.
96%andlinoleicacidfrom26.
34%to25.
41%,respec-tively.
Thepossiblereasonwasunsaturatedfattyacidswereunstableandoxidizedafterstoredforlongtime.
LinolenicacidwasnotdetectedbyGCafterstored1year.
Itcouldbeconcludedthatthebiodieselwasstableandqualiedforuse.
3.
5.
CatalystdeactivationDeactivationofsolidbasiccatalystforbiodieselproductionwascausedbythethreemainreasons.
Firstly,productsandby-prod-uctswereabsorbedonthesurfaceofthecatalystthatwoulddecreasethecontactbetweenbasicsitesandreactants.
Secondly,theactivitysiteswereleachedpartlyintosolutions.
Thelastreasonwasthatthecatalyststructurecollapsed.
Biodieselyieldwas95.
2%ifthecatalystwasrst-timeused.
Afterreaction,theusedcatalystwasseparatedbyhigh-speedcentrifugation,andreusedascatalystforthesecondtime.
Biodieselyieldwasdecreasedto90.
4%.
Forthethirdtimeused,biodieselyieldwasonly80.
7%.
Theservicelifeofthecalcinedhydrotalciteforbio-dieselproductionwasonly3times.
Afterthecatalystwassepa-rated,wefoundthatviscousliquidwasabsorbedonthesurfaceofthecatalyst.
FT-IR(FourierTransformInfrared)spectrum(Fig.
7)showedthattheliquidwasglycerolthatdeactivatedthecatalyst.
Therefore,theviscousliquidonthecatalystwasremovedbywashingwithethanol,andthecatalystwasreused.
Atthe8thtimereused,biodieselyieldwas89.
1%.
Butatthe9thtime,biodieselyieldsharplydecreasedtoabout43.
7%.
Thecatalystwasdeacti-vatedbutwithoutleachabilityofMg2andAl3intheliquidproductsasconrmedbyICP(InductivelyCoupledPlasma)[34].
SEMimageofthedeactivatedcatalystwasshowninFig.
8.
Thecatalysthadaplatelet-likestructure(402nmwidthand64nmthickness),butitwasnotasuniformastheinitialcalcinedhydrotalcite(Fig.
3B).
ItwasfoundthattheactivesitesofthedeactivatedcatalystdisappearedduetoitsstructurecollapsedidentiedbyXRD[35].
Table2PropertiesofJatrophabiodiesel.
ParameterJatrophaoilBiodieselTheGermanStandard(DINV51606:1997)Density(g/mL,289K)0.
8920.
8860.
875e0.
900Flashpoint(K)498459!
373Viscosity(mm2/s,313K)24.
53.
893.
5e5.
0Acidvalue(mgKOH/g)10.
50.
1540.
5Freemethanolcontent(wt%)e0.
20.
3Freeglycerolcontent(wt%)e0.
160.
2Totalglycerolcontent(wt%)e0.
180.
25Sulphurcontent(wt%)e0.
0030.
01Cetanenumber5158!
49Watercontent/(mg/kg)5200172300Ashcontent(wt%)e0.
0240.
05Pourpoint(K)271268eCarbonresidue(%)1.
00.
20.
05Copperstripcorrosion1a1a1aCaloricvalue(MJ/kg)38.
6541.
72eColoryellowbuffeTable3StabilityofJatrophabiodiesel.
ParameterBiodiesel(freshproduced)Biodiesel(storedfor1year)Density(g/mL,289K)0.
8860.
887Viscosity(mm2/s,313K)3.
894.
05Acidvalue(mgKOH/g)0.
1540.
36palmiticacid13.
79%15.
2%palmitoleicacid0.
95%0.
87%stearicacid6.
33%8.
37%oleicacid42.
61%38.
96%linoleicacid26.
34%25.
41%linolenicacid0.
06%eX.
Dengetal.
/Energy36(2011)777e7847824.
ConclusionSolidbasenanocatalystderivedfromhydrotalciteswithMg/Almolarratioof3/1wassynthesizedbycoprecipitationmethodusingureaasprecipitatorandwithMHT,followedbycalcination.
Thecatalystwasonly7.
3nmbycalculation,butaccordingtoAMFanalysis,theycongregatedtoformalayeredstructurewithsizeaslargeas0.
941-mmwidthand381-nmthickness.
Owingtoitsstrongbasicity,thecatalystwasusedforthetransestericationofJatrophaoilcon-taining5-12AVstobiodieselafterpretreatment.
Itwasfoundthatat210Wultrasonicpower,whenmethanolreactedwiththeoil(4/1molarratio)mixedwith1.
0wt%catalystat318Kfor1.
5h,biodieselyieldof95.
2%wasobtainedthatwashigherthanpreviousreported,thebiodieselpropertieswereclosetothoseoftheGermanstandard(DINV51606).
Themainreasonsforcatalystdeactivationwerethesurfaceabsorptionofby-productglycerolaswellasthecollapseofthelayeredstructure.
Afterremovingtheglycerolonthesurface,thecatalystwasreusedfor8times.
ItcouldbeconcludedthatthecalcinedhydrotalcitenanocatalystcombinedwithultrasonicradiationisaneffectivemethodfortheproductionofbiodieselfromJatrophaoil.
AcknowledgmentsTheauthorswishtoacknowledgethenancialsupportfromChineseAcademyofSciences[BairenJihuaandknowledgeinno-vationkeyproject(KSCX2-YW-G-075)],andChinaNationalScienceFoundation(No:21076220).
References[1]MaFR,HannaMA.
Biodieselproduction:areview.
BioresourceTechnology1999;70:1e15.
[2]PramanikK.
PropertiesanduseofJatrophacurcasoilanddieselfuelblendsincompressionignitionengine.
RenewableEnergy2003;28:239e48.
[3]DemirbasA.
Progressandrecenttrendsinbiodieselfuels.
EnergyConversionandManagement2009;50:14e34.
[4]MacorA,PavanelloP.
Performanceandemissionsofbiodieselinaboilerforresidentialheating.
Energy2009;34:2025e32.
[5]FernandoSD,KarraP,HernandezR,JhaSK.
Effectofincompletelyconvertedsoybeanoilonbiodieselquality.
Energy2007;32:844e51.
[6]ChenKS,LinYC,HsiehLT,LinLF,WuCC.
Savingenergyandreducingpollutionbyuseofemulsiedpalmbiodieselblendswithbio-solutionadditive.
Energy2010;35:2043e8.
[7]GuiMM,LeeKT,BhatiaS.
Feasibilityofedibleoilvs.
non-edibleoilvs.
wasteedibleoilasbiodieselfeedstock.
Energy2008;33:1646e53.
[8]GaoYY,ChenWW,LeiH,LiuY,LinX,RuanRS.
Optimizationoftrans-estericationconditionsfortheproductionoffattyacidmethylester(FAME)fromChinesetallowkerneloilwithsurfactant-coatedlipase.
BiomassBio-energy2008;33:277e82.
[9]YangCY,DengX,FangZ,PengDP.
SelectionofhighoilyieldseedsourcesofJatrophacurcasL.
forbiodieselproduction.
Biofuels2010;1:705e17.
[10]DengX,FangZ,LiuYH.
UltrasonictransestericationofJatrophacurcasL.
oiltobiodieselbyatwo-stepprocess.
EnergyConversionManagement2010;51:2802e7.
[11]OpenshawK.
AreviewofJatrophacurcas:anoilplantofunfullledpromise.
BiomassBioenergy2000;19:1e15.
[12]TamlampudiS,TalukderMR,HamaS,NumataT,KondoA,FukudaH.
Enzy-maticproductionofbiodieselfromJatrophaoil:acomparativestudyofimmobilizedwholecellandcommerciallipasesasbiocatalyst.
BiochemistryEngineeringJournal2007;39:185e9.
[13]AntolínG,TinautFV,BriceoY,CastaoV,PérezC,RamírezAI.
Optimizationofbiodieselproductionbysunoweroiltransesterication.
BioresourceTechnology2002;83:111e4.
[14]TanKT,LeeKT,MohamedAR.
Potentialofwastepalmcookingoilforcatalyst-freebiodieselproduction.
Energy2010;35:1e4.
[15]ZengHY,FengZ,DengX,LiYQ.
ActivationofMg-Alhydrotalcitecatalystsfortransestericationofrapeoil.
Fuel2008;87:3071e6.
[16]TantirungrotechaiJ,ChotmongkolsapP,PohmakotrM.
Synthesis,character-ization,andactivityintransestericationofmesoporousMg-Almixedmetaloxides.
MicroporousandMesoporousMaterials2010;128:41e7.
[17]XieWL,PengH,ChenLG.
CalcinedMg-Alhydrotalcitesassolidbasecatalystsformethanolysisofsoybeanoil.
JournalMolecularCatalysisA:Chemistry2006;246:24e32.
[18]BritoA,BorgesME,GarínM,HernándezA.
BiodieselproductionfromwasteoilusingMg-Allayereddoublehydrotalcitecatalysts.
Energy&Fuels2009;23:2952e8.
[19]DengX.
MicrostructurecontrollablepreparationandapplicationofNano-crystallineMg-Alhydrotalcite,MSc.
Thesis(inChinese),DepartmentofChemicalEngineering,XiangtanUniversity,China;2007,p.
77e86.
[20]FraileJM,GarcíaN,MayoralJA.
TheinuenceofalkalinemetalsonthestrongbasicityofMg-Almixedoxides:thecaseoftransestericationreactions.
AppliedCatalysisA:General2009;364:87e94.
[21]FangF.
Thepreparationofbiodieselandthestudiesonits'analyticalmethodofqualityindex,MSc.
Thesis(inChinese),DepartmentofChemicalEngi-neering,XiangtanUniversity,China;2005,p.
44e60.
[22]AramendíaMA,BorauV,JiménezC,MarinasJM,RuizJR,UrbanoFJ.
Catalytichydrogentransferfrom2-propanoltocyclohexanoneoverbasicMg-Aloxides.
AppliedCatalysisA:General2003;255:301e8.
[23]BirksLS,FriedmanH.
ParticlesizedeterminationfromX-raylinebroadening.
JournalofAppliedPhysics1946;17:687e91.
[24]FangZ,AssaaoudiH,GuthrieRIL,KozinskiJA,ButlerIS.
Continuoussynthesisoftinandindiumoxidenanoparticlesinsub-andsupercriticalwater.
JournalAmericanCeramicSociety2007;90:2367e71.
[25]KusˇtriwskiP,ChmielarzE,BozekE,SawalhaM,RoessnerF.
AcidityandbasicityofhydrotalcitederivedmixedMg-AloxidesstudiedbytestreactionofMBOHconversionandtemperatureprogrammedde-sorptionofNH3andCO2.
JournalMaterialsResearchBulletin2004;39:263e81.
[26]XieWL,LiH.
Alumina-supportedpotassiumiodideasaheterogeneouscata-lystforbiodieselproductionfromsoybeanoil.
JournalMolecularCatalysisA:Chemistry2006;255:1e9.
[27]BarakosN,PasiasS,PapayannakosN.
TransestericationoftriglyceridesinhighandlowqualityoilfeedsoveranHT2hydrotalcitecatalyst.
BioresourceTechnology2008;99:5037e42.
Fig.
8.
SEMimageofthedeactivatedcatalyst.
Fig.
7.
FT-IRspectrumoftheviscousliquidonthesurfaceoftheusedcatalyst.
X.
Dengetal.
/Energy36(2011)777e784783[28]LeungDY,GuoY.
Transestericationofneatandusedfryingoil:optimizationforbiodieselproduction.
FuelProcessingTechnology2006;87:83e90.
[29]LiuXH,XiongXY,LiuCM,LiuDY,WuAJ,HuQL,etal.
PreparationofbiodieselbytransestericationofrapeseedoilwithmethanolusingsolidbasecatalystcalcinedK2CO3/g-Al2O3.
JournalofAmericanOilChemistsSociety2010;87:817e23.
[30]HinguSM,GogatePR,RathodVK.
Synthesisofbiodieselfromwastecookingoilusingsonochemicalreactors.
UltrasonicsSonochemistry2010;17:827e32.
[31]JainS,SharmaMP.
ProspectsofbiodieselfromJatrophainIndia:areview.
RenewableandSustainableEnergyReviews2010;14:763e71.
[32]DeOliveiraJS,LeitePM,DeSouzaLB,MelloVM,SilvaEC,RubimJC,etal.
CharacteristicsandcompositionofJatrophagossypiifoliaandJatrophacurcasL.
oilsandapplicationforbiodiesel.
BiomassandBioenergy2009;33:449e53.
[33]TiwariAK,KumarA,RahemanH.
BiodieselproductionfromJatrophaoil(Jatrophacurcas)withhighfreefattyacids:anoptimizedprocess.
BiomassandBioenergy2007;31:569e75.
[34]LiuXJ,PiaoXL,WangYJ,ZhuSL,HeHY.
Calciummethoxideasasolidbasecatalystforthetransestericationofsoybeanoiltobiodieselwithmethanol.
Fuel2008;87:1076e82.
[35]GaoLJ,TengGY,LvJH,XiaoGM.
BiodieselsynthesiscatalyzedbytheKF/Ca-Mg-Alhydrotalcitebasecatalyst.
EnergyFuels2010;24:646e51.
X.
Dengetal.
/Energy36(2011)777e784784
- sitesyw372相关文档
- frequencyyw372
- responyw372
- 消费yw372
- shigayw372
- 公司yw372
- 南乐县yw372
Bluehost美国虚拟主机2.95美元/月,十八周年庆年付赠送顶级域名和SSL证书
Bluehost怎么样,Bluehost好不好,Bluehost成立十八周年全场虚拟主机优惠促销活动开始,购买12个月赠送主流域名和SSL证书,Bluehost是老牌虚拟主机商家了,有需要虚拟主机的朋友赶紧入手吧,活动时间:美国MST时间7月6日中午12:00到8月13日晚上11:59。Bluehost成立于2003年,主营WordPress托管、虚拟主机、VPS主机、专用服务器业务。Blueho...
ATCLOUD-KVM架构的VPS产品$4.5,杜绝DDoS攻击
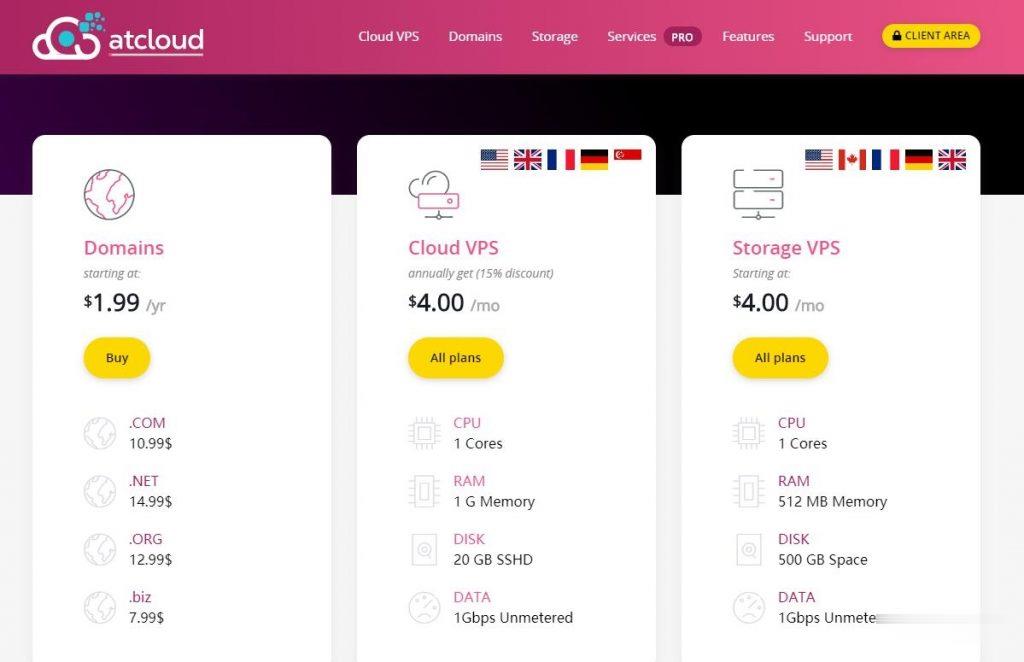
ATCLOUD.NET怎么样?ATCLOUD.NET主要提供KVM架构的VPS产品、LXC容器化产品、权威DNS智能解析、域名注册、SSL证书等海外网站建设服务。 其大部分数据中心是由OVH机房提供,其节点包括美国(俄勒冈、弗吉尼亚)、加拿大、英国、法国、德国以及新加坡。 提供超过480Gbps的DDoS高防保护,杜绝DDoS攻击骚扰,比较适合海外建站等业务。官方网站:点击访问ATCLOUD官网活...
wordpress专业外贸建站主题 WordPress专业外贸企业网站搭建模版
WordPress专业外贸企业网站搭建模版,特色专业外贸企业风格 + 自适应网站开发设计 通用流行的外贸企业网站模块 + 更好的SEO搜索优化和收录 自定义多模块的产品展示功能 + 高效实用的后台自定义模块设置!采用标准的HTML5+CSS3语言开发,兼容当下的各种主流浏览器: IE 6+(以及类似360、遨游等基于IE内核的)、Firefox、Google Chrome、Safari、Opera...

yw372:Com为你推荐
-
支持ipad关键词网易yeah360退出北京时间北京时间校准显示时间重庆电信断网这几天为什么重庆电信的网络总是这么不稳定人人视频总部基地落户重庆迁户口入重庆netshwinsockreset在cmd中输入netsh winsock reset显示系统找不到指定文件怎么办degradeios传奇域名自己的传奇服务器怎么建设?科创板首批名单首批公布的24个历史文化明城是那些zhuo爱大涿爱— 金鱼花火 、 歌词给我翻译过来。!